RUDN University chemists proposed a way to reduce three times the temperature for the oxidation of alkanes
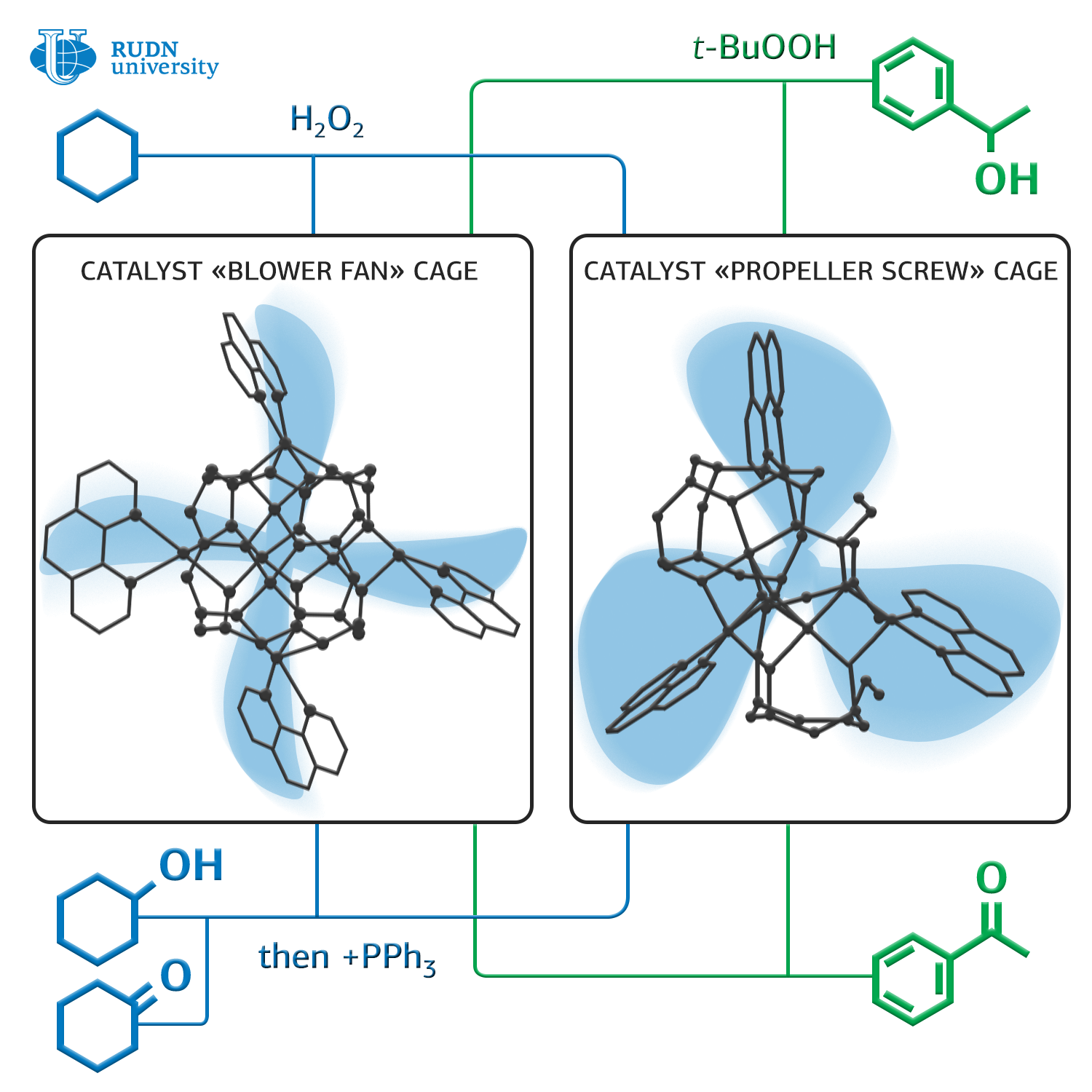
Until now, the reaction of catalytic oxidation of alkanes to alcohols, aldehydes, ketones, carboxylic acids could be carried out only at high temperatures — 150 degrees Celsius and above. Lowering the process temperature will simplify the synthesis and significantly reduce the cost of the final products. But this requires new catalysts.
Chemists Georgy Shulpin and Alexey Bilyachenko from RUDN University together with colleagues from the Russian Academy of Sciences synthesized two new organoelemental compounds with a framework structure that can significantly reduce the oxidation temperature of alkanes — from 150 to 50 degrees Celsius.
These catalysts are based on silsesquioxanes — polymeric compounds with the general formula [RSiO3/2]n, (sesqui in Latin means “one and a half”). For their synthesis, a simple two-stage scheme is used. The first structure is formed in tetrahydrofurane and contains Cu4Na4, the second in dimethylformamide and contains Cu5. RUDN University chemists studied the molecular structure of the obtained compounds, and also a structure of the supramolecular structures formed by them in crystals.
The researchers tested the catalytic activity of these compounds using them as catalysts for the oxidation reaction of hexalnethylene to cyclohexanol and cyclohexanone under the action of hydrogen peroxide in acetonitrile at 50 degrees. The conversion — the ratio of the amount of the obtained product to the theoretically possible amount — was about 25% in this reaction, which is comparable to the indicators of the traditional high-temperature method. In addition, chemists used these catalysts in the oxidation reaction of cyclohexanol to cyclohexanone and 1-phenylethanol to acetophenone under the action of tert-butyl hydroperoxide at the same temperature. The conversion in the case of cyclohexanol was about 40%, and the oxidation of 1-phenylethanol to acetophenone was almost complete. Thus, chemists took an important step to simplify the technology of the synthesis of a number of important compounds for industry.
Chemists emphasize that the production of cyclohexanone from hexalnethylene is important since from cyclohexanone adipic acid is obtained — the raw material for the production of nylon-6,6, and caprolactam — the raw material for the production of capron (nylon-6). Now in the industry, the process of synthesis of cyclohexanone from hexalnethylene is carried out at a temperature of about 150 degrees; the new method will reduce the temperature to 50 degrees.
The article is published in the journal Catalysts.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.