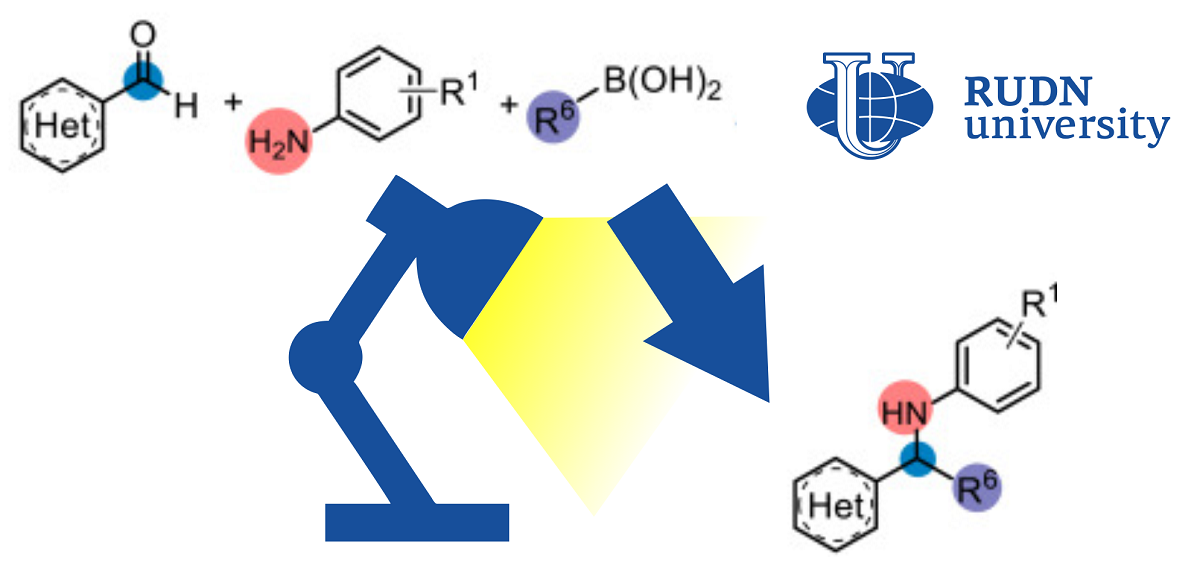
RUDN Chemist Improves Classical Reaction to Produce Complex Amines
In classical chemical reactions, only two initial substances are involved. Modern chemistry is increasingly based on the so-called multicomponent reactions, in which several substances are combined into the final product. This greatly expands the possibilities of synthesis. One such approach, the Petasis reaction, combines amines, aldehydes, and acids to produce more complex amines. The Petasis reaction is used, for example, in the pharmaceutical and agrochemical industries. However, the possible scope of use is limited due to the nature of the process — for the “transportation” of one fragment to another, boric acid salts (borates) must be formed. The fact is that the classical Petasis reaction follows the scheme of two-electron transfer, when two electrons must move from one fragment to another. Because of this, the original compounds have a limited set of suitable functional groups.
“Despite the synthetic utility of the reactionand Petawithisa, the shortcomings of the traditional two-electron circuitry continue to stimulate new research. In this regard, we present the use of free boric acid derivatives, which are effective in the donorofradicals using the mechanism of single-electron transport under mild reaction conditions,” Professor Eric Van der Eyken,Head of the Joint Institute chemical research of RUDN University.
Chemists improved the classical protocol with boric acid derivatives and light. For successful accession, the formation of borates is not necessary — the peaktion goes in soft conditions in visible light.
To find the optimal conditions, chemists conducted a series of experiments. In a solution of dimethylformamide at a temperature of 30 ° C and blue light (400 nm) in 24 hours, it was possible to achieve a 76%yield of the final amine. With the same conditions, chemists tested the initial compounds with other functional groups and obtained more than 30 products with an output of up to 95%. Convinced of the effectiveness and wide applicability of the method, chemists tested it on a semi-industrial scale — in a continuous reactor. For at least three final products, this allowed to achieve a significant increase in yield — from 31-76% to 85-90%, while the reaction time was reduced from 24 hours to 50 minutes.
“The oxidative potential of boric acid derivatives was regulated by hydrogen bonding using dimethylformamide as a solvent. We also studied the process in the continuous reactorto make it easier to scaleand shortenthe reactionwhile increasing its efficiency. universality from the point of view of functional groups and is suitable for obtaining complexand secondaryamines from simple predecessors,” Professor Eric Van der Eyken,Head of the Joint Institute for Chemical Research, RUDN University.
The results are published in the journal iScience.
Products derived from microalgae represent a cutting-edge development in the field of bioeconomy. The potential of this biological resource was discussed at the international research seminar “Foundations for a Green Sustainable Energy”, part of the BRICS Network University’s thematic group on “Energy”. The event was organized by the Institute of Ecology at RUDN University.
Ambassadors of Russian education and science met at a conference in RUDN University to discuss how they can increase the visibility of Russian universities and research organizations in the world, and attract more international students in Russia.
The international scientific seminar hosted by RUDN Institute of Ecology “Experience of participation in student organizations as a way to form career skills” united scholarship recipients of the International Student Mobility Awards 2024 and Open Doors, along with members of the scientific student society “GreenLab” and the professional student association “Kostyor (Bonfire)” shared their projects focused on environmental protection.