RUDN chemists have created a “green” catalyst for the synthesis of complex molecules for medicine and industry
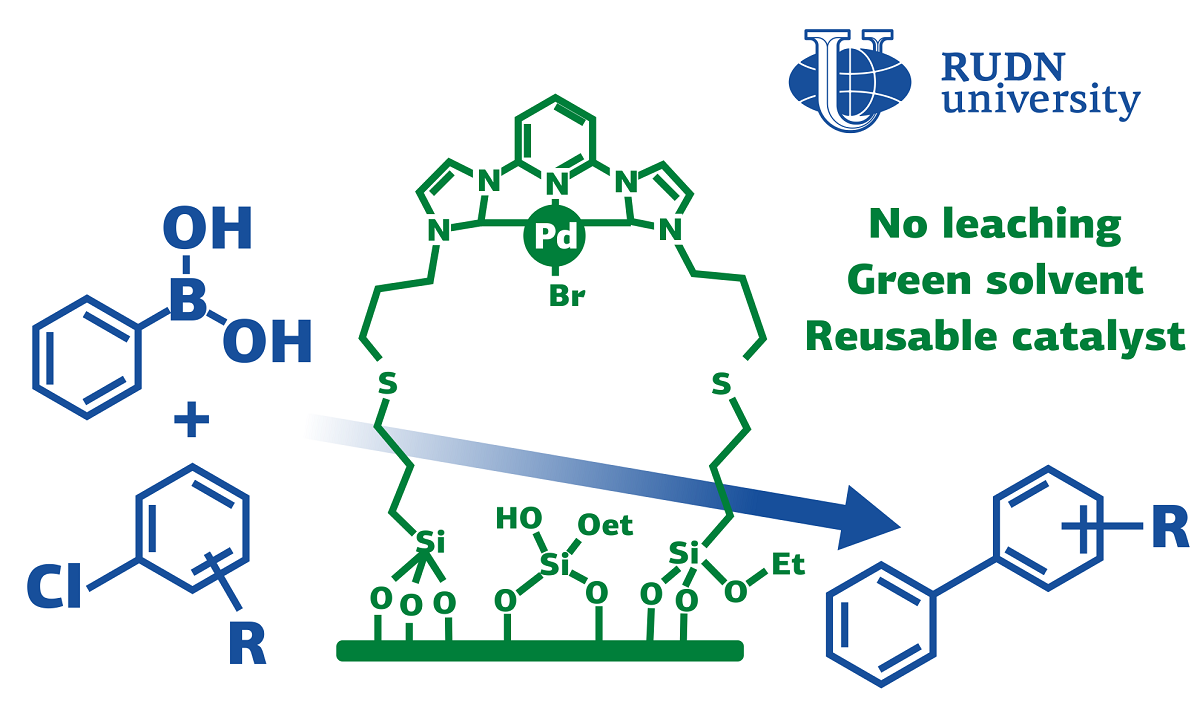
For the synthesis of complex molecules for pharmaceuticals, agrochemistry and the chemical industry, cross-combination reactions are used — compounds of two carbon atoms from different molecules. One of the cross-combination reactions is the Suzuki reaction. It requires palladium-based catalysts. The noble metal allows the reaction to be carried out effectively, but it can “wash out” and get into the resulting product. This is especially undesirable, for example, in the production of medicines.
“To stop the leaching of metal during the catalytic cycle, we propose to use organometallic compounds. The strong interaction between organic bonds and metals forms highly stable complexes. This effective interaction helps to keep the metal from leaching, ” Professor Leonid Voskresensky, Dean of the Faculty of Physics, Mathematics and Natural Sciences of the RUDN.
RUDN chemists have created a palladium nanocatalyst based on N-heterocyclic carbenes (NHC). After use, it leaves no traces of palladium in the product. The catalyst proved to be efficient and economical for the Suzuki reaction between phenylboric acid and aryl chlorides. The reaction took place at a temperature of 90 ℃ for eight hours. After that, the solid residue was washed in water and ethyl acetate, dried and the reaction was carried out again to check how the structure and effectiveness of the catalyst changed. The chemists also tested different reaction conditions — temperature, solvent and base— to find the optimal ones.
After separating the catalyst, chemists performed a spectroscopic analysis of the filtrate and made sure that it did not contain palladium. The maximum yield of the final product was 97% for one of the aryl chlorides. This is comparable to the results of other catalysts, but, unlike analogues, the catalyst consumption was less. In addition, a “green” solvent was used — an aqueous solution of isopropanol. After 10 cycles of use, the efficiency of the catalyst has practically not changed, remaining above 90%. Moreover, after the ninth use, chemists checked the structure of the nanocatalyst using a scanning electron microscope and found out that it remained almost the same as it was before the first use.
“The hybrid nanomaterial acts as a highly active catalyst in the Suzuki reaction between aryl chlorides and phenylboric acid. The catalyst has shown excellent activity and stability, it can be reused at least ten times without any significant loss of activity. After the ninth launch, the catalyst showed an almost similar structure compared to the fresh catalyst. We found no traces of palladium in the products, and leaching tests confirmed that the reaction was really heterogeneous,” — Rafael Luque, professor at the Center for Molecular Design and Synthesis of Innovative Compounds for Medicine of the RUDN.
The results are published in the journal Molecular Catalysis
Products derived from microalgae represent a cutting-edge development in the field of bioeconomy. The potential of this biological resource was discussed at the international research seminar “Foundations for a Green Sustainable Energy”, part of the BRICS Network University’s thematic group on “Energy”. The event was organized by the Institute of Ecology at RUDN University.
Ambassadors of Russian education and science met at a conference in RUDN University to discuss how they can increase the visibility of Russian universities and research organizations in the world, and attract more international students in Russia.
The international scientific seminar hosted by RUDN Institute of Ecology “Experience of participation in student organizations as a way to form career skills” united scholarship recipients of the International Student Mobility Awards 2024 and Open Doors, along with members of the scientific student society “GreenLab” and the professional student association “Kostyor (Bonfire)” shared their projects focused on environmental protection.