RUDN chemists have found an effective catalyst for the synthesis of raw materials of the chemical industry
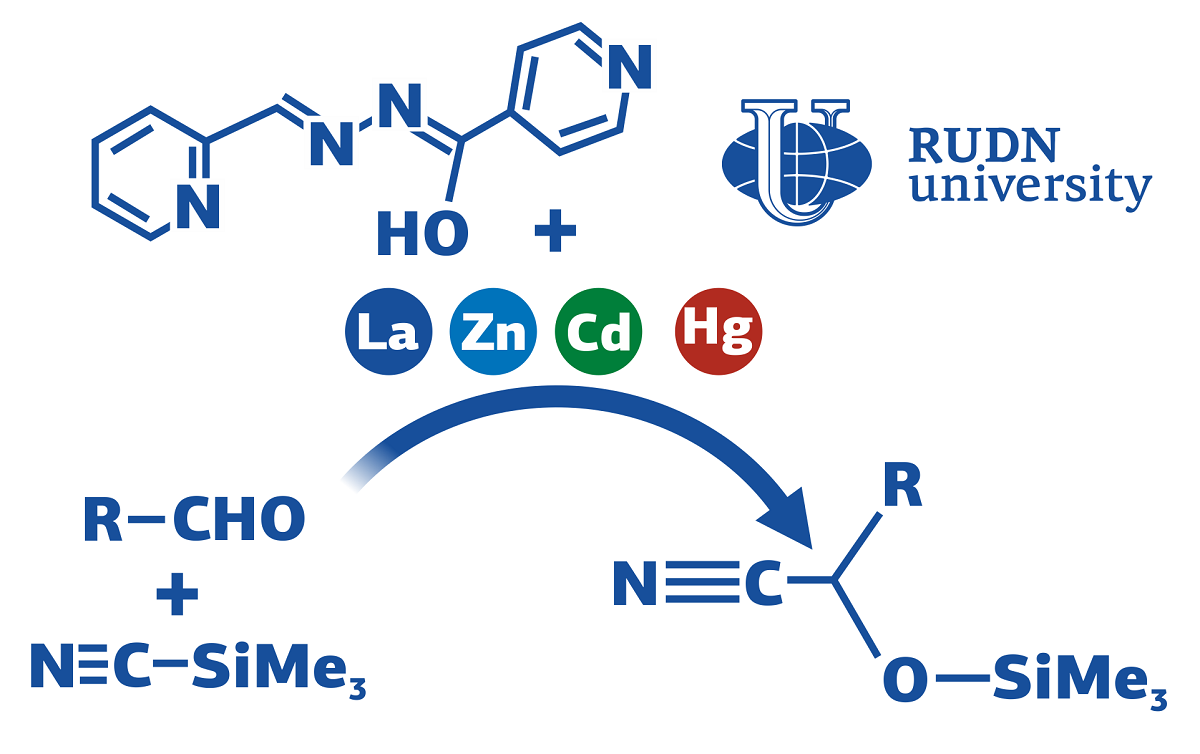
Cyanhydrins are organic substances with nitrile and hydroxyl groups that are used in industrial chemistry as raw materials for polymers, acids, alcohols and other compounds. One of the ways to obtain cyanhydrins is the addition of prussic acid to aldehydes. Without catalysts, this reaction is possible, but gives a small yield of the product — about 20%. To create existing catalysts, expensive and dangerous reagents are required, and the reaction itself takes time. RUDN chemists together with colleagues have shown that some metal complexes, the synthesis of which takes place under mild conditions, can be effective catalysts for this process.
“Despite the wide variety of catalytic systems for cyanhydrin production, most of them have significant disadvantages — the use of harmful and expensive components and solvents, long reaction time, low yields, etc. Therefore, the development of a practical catalytic system for the efficient production of cyanhydrins from aldehydes under mild conditions remains a difficult task. We decided to test several relatively easy-to-prepare and inexpensive metal complexes as potential reaction catalysts,” Candidate of Chemical Sciences Evgenia Nikitina, Associate Professor of the Department of Organic Chemistry of the RUDN.
For the experiment, chemists chose four complexes based on cadmium, mercury, zinc and lanthanum. Ligands — ligaments connecting the metal center and lateral branches — in all four cases were the same organic compound. Solvent, aldehyde and trimethylsilyl cyanide were added to the test tube with the catalyst, then the solution was mixed. The course of the reaction was monitored using thin-layer chromatography. After the reaction, the RUDN chemists examined the resulting product using NMR spectroscopy.
The most effective catalyst was a complex with lanthanum. In his presence, the reaction lasted only six hours at room temperature. The output of the final product was close to ideal — 96.3%. Chemists also studied which components of the lanthanum catalyst are responsible for its effectiveness, and came to the conclusion that both non-covalent interactions and donor-acceptor bonds are crucial. The first — weaker — determine the shape and stability of the molecule, the second — stronger — hold its very structure.
“Compared to other coordination catalysts for similar aldehyde conversion, the lanthanum-based catalyst has better performance. The yield of the product is up to 96.3% in six hours at room temperature. Moreover, in previously studied catalytic systems based on metal complexes, additives, a longer reaction time, a larger amount of catalyst and more extreme temperatures are required, while the yield of cyanhydrins is usually lower,” — Candidate of Chemical Sciences Fedor Zubkov, Associate Professor of the Department of Organic Chemistry of the RUDN.
The results are published in the journal Polyhedron.
Products derived from microalgae represent a cutting-edge development in the field of bioeconomy. The potential of this biological resource was discussed at the international research seminar “Foundations for a Green Sustainable Energy”, part of the BRICS Network University’s thematic group on “Energy”. The event was organized by the Institute of Ecology at RUDN University.
Ambassadors of Russian education and science met at a conference in RUDN University to discuss how they can increase the visibility of Russian universities and research organizations in the world, and attract more international students in Russia.
The international scientific seminar hosted by RUDN Institute of Ecology “Experience of participation in student organizations as a way to form career skills” united scholarship recipients of the International Student Mobility Awards 2024 and Open Doors, along with members of the scientific student society “GreenLab” and the professional student association “Kostyor (Bonfire)” shared their projects focused on environmental protection.