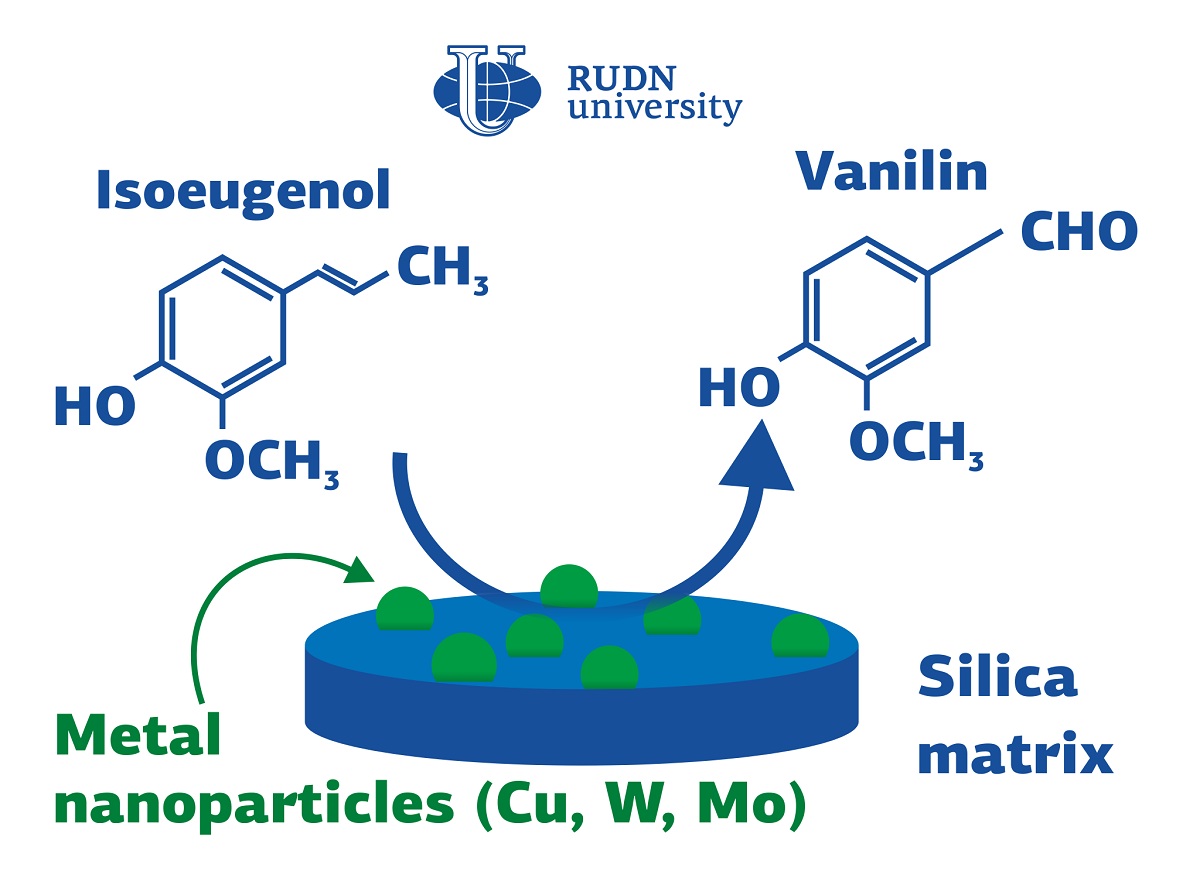
RUDN University chemist creates nanocatalysts for vanillin synthesis
Only 1% of the annually produced worldwide 20 thousand tons of vanillin is made from natural vanilla. Almost all vanillin in seasonings, pastries, pharmaceuticals and cosmetics is synthesized by chemical protocols. Usually, petrochemical raw materials are used for this, but synthesis from inexpensive plant biomass is also possible. The main ingredient is lignin. This polymer is widely available as it is part of the trees, and it is obtained in the production of paper as a by-product. It is easy to isolate eugenol and other substances suitable for the synthesis of vanillin from lignin, but the next step is challenging. In oxidation reactions, along with vanillin, several by-products similar to it in structure are formed. It is difficult to separate them. The RUDN University chemist proposed a number of eco-friendly nanocatalysts that will allow obtaining more vanillin from plant raw materials than traditional methods.
“For many years, the chemical industry has been interested in new catalysts to increase the economic efficiency of production. Today, catalysts that allow switching to more environmentally friendly methods of chemical synthesis are in demand. The higher their catalytic activity, the milder the conditions in which we can apply them. This is a chance to reduce energy costs and the burden on the environment”, said Prof. Rafael Luque from the Joint Institute for Chemical Research, RUDN University.
To create an effective catalyst, the RUDN chemist used the advantages of nanostructured materials. The smaller pores and channels per unit volume of the catalyst, the larger the surface with which chemicals can interact during the reaction. But the active nanoparticles must be securely bound to this surface so as not to be washed out at the first use of the catalyst. The solution was found in the combination of substances of different classes: silicon base and metal nanoparticles. To do this, a metal salt and tetraethoxysilane (a precursor of silicon dioxide) were mixed in a solvent and the mixture was exposed to microwaves at a temperature of 80-100°C for several minutes. In a series of experiments, different metals were used: copper, niobium, molybdenum, and tungsten. All the materials turned out to be highly porous after drying. Microwaves caused the formation of nanotubes in them. Additional studies have shown that metal nanoparticles are embedded in the silicon matrix.
All the obtained catalysts were tested in the reaction of oxidation of isoeugenol with hydrogen peroxide. The reagents were combined in a solvent at a temperature of 80°C for 6 hours. During this time, all materials, except for the catalyst with molybdenum, provided the processing of more than 50% of isoeugenol. But the high selectivity with respect to vanillin, that is, the production of the largest proportion of this substance, was provided only by a catalyst with copper. In his case, 88% of the reaction products were vanillin.
“The synthesized catalysts possess a significant potential as catalyst in the wet oxidation of isoeugenol to vanillin. One of the obtained materials with the inclusion of copper showed significantly higher selectivity than previously known catalysts. As repeated reactions have shown, this catalyst can be used up to four times without significant loss of efficiency”, said Prof. Rafael Luque from the Joint Institute for Chemical Research, RUDN University.
The results of the study are published in Molecular Catalysis.
The RUDN Prize for Scientific Achievements in Chemistry for 2025, with a monetary award of 2 million rubles, was awarded to Alexander Davidovich Dilman, Deputy Director of the N.D. Zelinsky Institute of Organic Chemistry of the Russian Academy of Sciences. The researcher received the award during the celebration marking RUDN’s 66th anniversary.
Sergey Ivanov, a scholar from St. Petersburg, has been named the first winner of RUDN University’s International Prize for Scientific Achievements in Mathematics, worth 5 million rubles.
Products derived from microalgae represent a cutting-edge development in the field of bioeconomy. The potential of this biological resource was discussed at the international research seminar “Foundations for a Green Sustainable Energy”, part of the BRICS Network University’s thematic group on “Energy”. The event was organized by the Institute of Ecology at RUDN University.