Scientific seminar of post-doc Efimov I.V. «Novel and convenient route toward 2,4-disubstituted pyrroles based on the reaction of enaminones and isocyanides»
Pyrrole core is the basis of many pharmacologically active compounds1,2. For example, 3-substituted pyrroles are found in a variety of medicinal and agrochemical applications, such as efficient inhibitors of histone deacetylase3, HIV-1 transcriptase4, and COX-1/COX-2 cyclooxygenases5. Furthermore, the 4-acetyl pyrroles (JWH series) has moderate affinity for the CB1 receptor6. Besides a wide range of applications in medicinal chemistry pyrroles are also found to be electronically polarizable and oxidizable building blocks for polymeric and supramolecular structures for applications in nonlinear optics7.
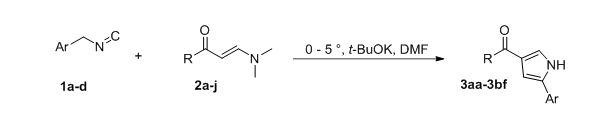
Herein, we suggest novel and convenient rout toward 2,4-disubstituted pyrroles based on the reaction aromatic enaminones and isocyanides, having active methylene group. Our experiments has show that the best reaction conditions include using potassium tert-butoxide as base and DMF as solvent at 0-5 °C. It was found, that reaction was finished immediately after addition all amount of isocyanide. Finally, 30 new 4-acetylpyrrole were synthesized and fully characterized by complex of spectral methods of analysis and X-ray structural analysis.