RUDN chemist obtained a reusable catalyst for the synthesis of esters
An enlarged model of spacecraft systems and specific characteristics reflecting the current level of technology (for example, the ratio of the solar panel mass to electric power) were used to determine these parameters.
The researchers reviewed considered missions to Mars and Mercury. Calculations have shown that the space probe with EPS and with the specified characteristics will be able to reach Mars in 350 days at the start date of April 30, 2035. The flight transfer to Mercury will take about 3000 days.
Besides, mathematicians have shown that for a wide class of trajectories, the maximum value of useful spacecraft mass is achieved on the trajectory with the constantly running engine, that is, with the minimum possible thrust necessary for the flight.
"This suggests that the increase in thrust, which will reduce fuel costs, is ineffective in comparison with the increase in the required mass of the propulsion system itself. This is due to the main problem of space exploration – the lack of compact and powerful energy sources", — Alexey Ivanyukhin explains.
He and his colleagues are planning to continue research in this direction.
"For example, we intend to consider missions to asteroids to deliver soil or flights to the moon. It is possible to specify consider more detailed the model of operation of the EPS or solar panels. Developers of EPS and spacecrafts are interested in such studies", — Alexey Ivanyukhin concludes.
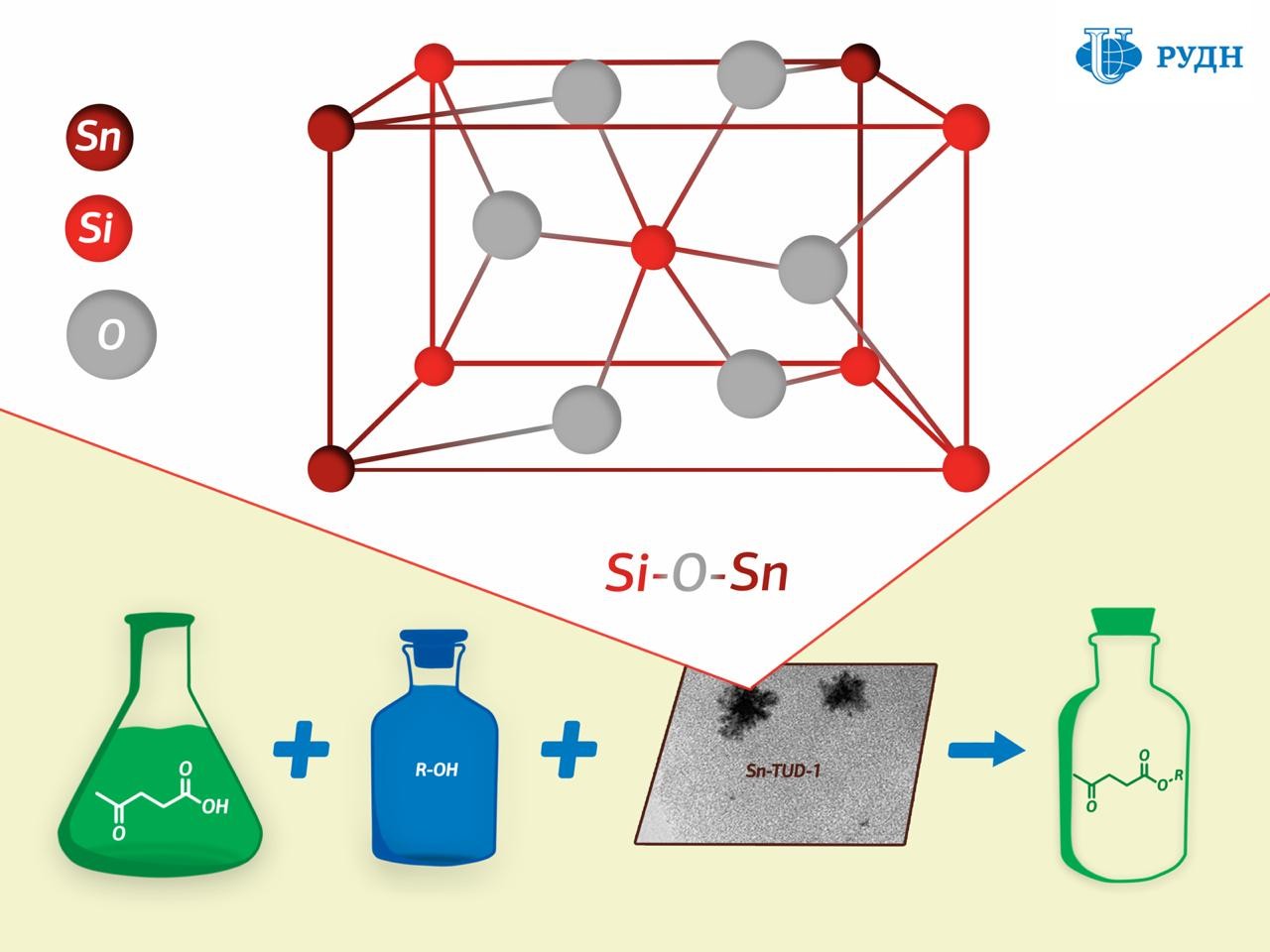
A chemist from RUDN University has developed a tin silicate catalyst for the production of esters — flavours, plasticisers, and biofuel components. Unlike existing catalysts, the new material can be made active again and reused. The results are published in the journal Microporous and Mesoporous Materials.
The catalysts are not consumed in the process of chemical reactions, yet it is difficult in some cases to separate them from the synthesis waste and reuse. For instance, inorganic acid catalysts are used for esterification, i.e. to obtain esters from organic acids and alcohol. In this case, the final product of reaction must be purified and the waste disposed of, along with the catalysts, since it is more expensive to separate them for reuse than to acquire new ones.
One promising solution is solid catalysts based on tin ions deposited on a porous support substrate. Its “active centres” are located on its surface: ions on which a chemical transformation occurs, for example, the formation of ether. However, tin ions are “washed out” during the use of such materials, and they lose their activity. Moreover, a lot of useless tin oxide is formed during the manufacture of the catalyst, in addition to ions.
RUDN University chemist Rafael Luque has developed a new catalyst production method that results in a porous silicate matrix with “embedded” tin ions Sn4+ held together by strong chemical bonds.
While the existing methods for creating such catalysts involve tin being applied to a finished porous matrix of silicon dioxide, professor Luque formed the catalyst “from scratch”. The silicon dioxide substrate in his experiment was formed from a precursor (tetraethoxysilane) in the presence of tin, due to which tin ions were embedded in the chemical structure of the substrate.
The study of the substrate using XPS X-ray photoelectron spectroscopy showed that a chemical bond of silicon oxide and tin (Si–O–Sn) indeed formed in the catalyst.
The surface area of 1 gram of catalyst is significant — it’s 600 square metres. Since chemical reactions occur on the surface of a catalyst, the larger its surface area, the higher the activity. Most catalysts based on a silicon matrix have a useful area two to three times smaller: about 200-300 square metres per gram.
Chemists tested the activity of the new catalyst in the synthesis of levulinic acid esters. Levulinic acid is a product of the processing of carbohydrates such as glucose and starch. When interacting with alcohols it forms esters, which can be used as flavorings, plasticisers, and components of biofuels. It turned out that the new catalyst allows obtaining esters of levulinic acid with a maximum product yield of 44 to 99 percent - the figure corresponds to the efficiency of most commonly used catalysts.
In addition, the catalyst was tested for “reusability”: the experiment showed that its activity did not decrease after five regenerations.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.