RUDN University Chemist Invented a "Line Production" Method of Synthesis of 28 Biologically Active Molecules
The search for new medications is usually initiated not by doctors, but by synthetic chemists: they obtain so-called libraries – a number of similar organic substances that can potentially exhibit biological activity. Dealing not with “individual” substances, but with libraries makes it easier to not overlook a highly effective compound, which can be only slightly different from its less effective analogues, “neighbours” in the library.
“Diverse small molecular libraries constitute a significant part in the drug/lead discovery process. Over the past decades, the development of high-throughput screening enabled the extremely rapid biological evaluation of large collections of small organic molecules. However, growing evidence now suggests that many existing compound collections are inadequate in the search for new molecular entities capable of interacting with complex biological targets. Thus, it is still in high demand to generate diverse molecular libraries”, says the author of the study, RUDN University chemist Erik Van der Eycken.
There are difficulties with creating some such libraries, e.g., for polycyclic molecules with several heteroatoms - nitrogen, oxygen, or sulphur. Most of the known compounds from this group exhibit biological activity: for instance, it includes vincamine, a drug for stimulating cerebral circulation in dementia, or tsitsikammamin, a topoisomerase inhibitor, known for its antimicrobial and antimalarial effect. However, there is no universal method for obtaining these compounds, and in order to verify their pharmacological action, it is necessary to come up with a particular and often multi-stage and expensive synthesis scheme for each of them.
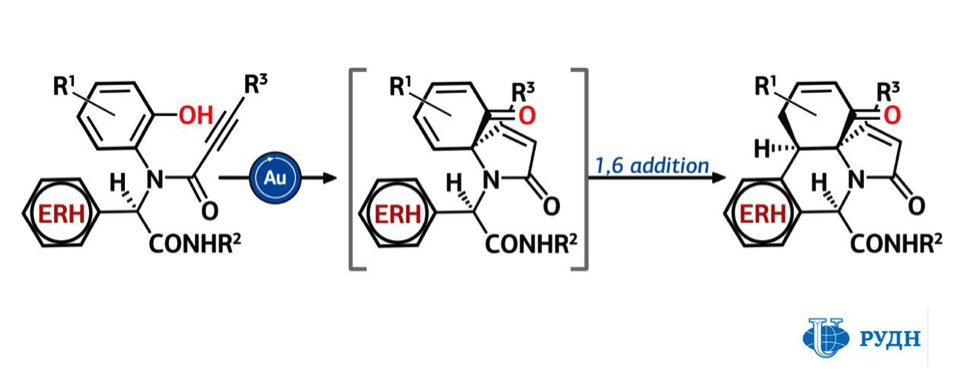
Van der Eycken came up with a method that allows obtaining compounds from this group using only widespread and cheap reagents. To achieve this, the chemist chose the multicomponent Ugi reaction for the first stage of the scheme. This reaction allows you to combine four simple organic molecules into a single structure. It helped Van der Eycken to obtain an aromatic polycyclic molecule with several heteroatoms out of indole, pyrrole, benzothiophene, or furan.
The next stage in the proposed scheme was the dearomatisation reaction: the saturation of double bonds in the benzene ring, combined with spirocyclisation, i.e. the formation of ring structures inside the molecule in which one carbon atom belongs to several cycles at once. It was the main stage and, unlike the first stage, it was used for such compounds for the first time.
The RUDN University chemist managed to select the conditions — the catalyst and the temperature — under which aromaticity is broken in aromatic heterocyclic structures, and sites from the opposite end of the complex molecule are attached to several carbon atoms. In total, the chemist checked 15 catalysts with similar composition based on gold chloride, and one of them made possible a yield of 53 to 98 percent, depending on the structure of reacting substances. The optimal synthesis temperature was from 70 to 110 degrees, and the synthesis time was from 2 to 30 hours.
It turned out that the proposed scheme is best used for obtaining compounds in which the rings either do not contain side groups at all, or these groups have an aromatic structure. However, since the acetyl side group generally stops the reaction, it can be used as a protective element in such a scheme. In total, the chemist synthesized 28 different compounds, each of which has the necessary polycyclic structure, which allowed to significantly expand the library of candidates for medications and biologically active substances.
The work was published in Organic & Biomolecular Chemistry
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.