A chemist from RUDN University and TIPS RAS analysed a new way to make heavy-duty polyethylene
Polyethylene is a network of linked ethylene chains. The longer the chains in the composition of polyethylene, the more stable the resulting network of polyethylene to stress and tension. Such properties are needed, for example, for the production of bioprostheses. Ethylene chains are obtained during the polymerisation reaction, which is characterised by a stepwise growth of ethylene molecules at the extending end of each polyethylene chain. The polymerisation reaction occurs thanks to an initiating agent and a catalyst, which results in the growth of ethylene chains.
The authors of the paper proposed changing the chemical composition of substances involved in the reactions in order to synthesise longer ethyl chains, which would mean stronger polyethylene, without resorting to an increase in the reaction temperature.
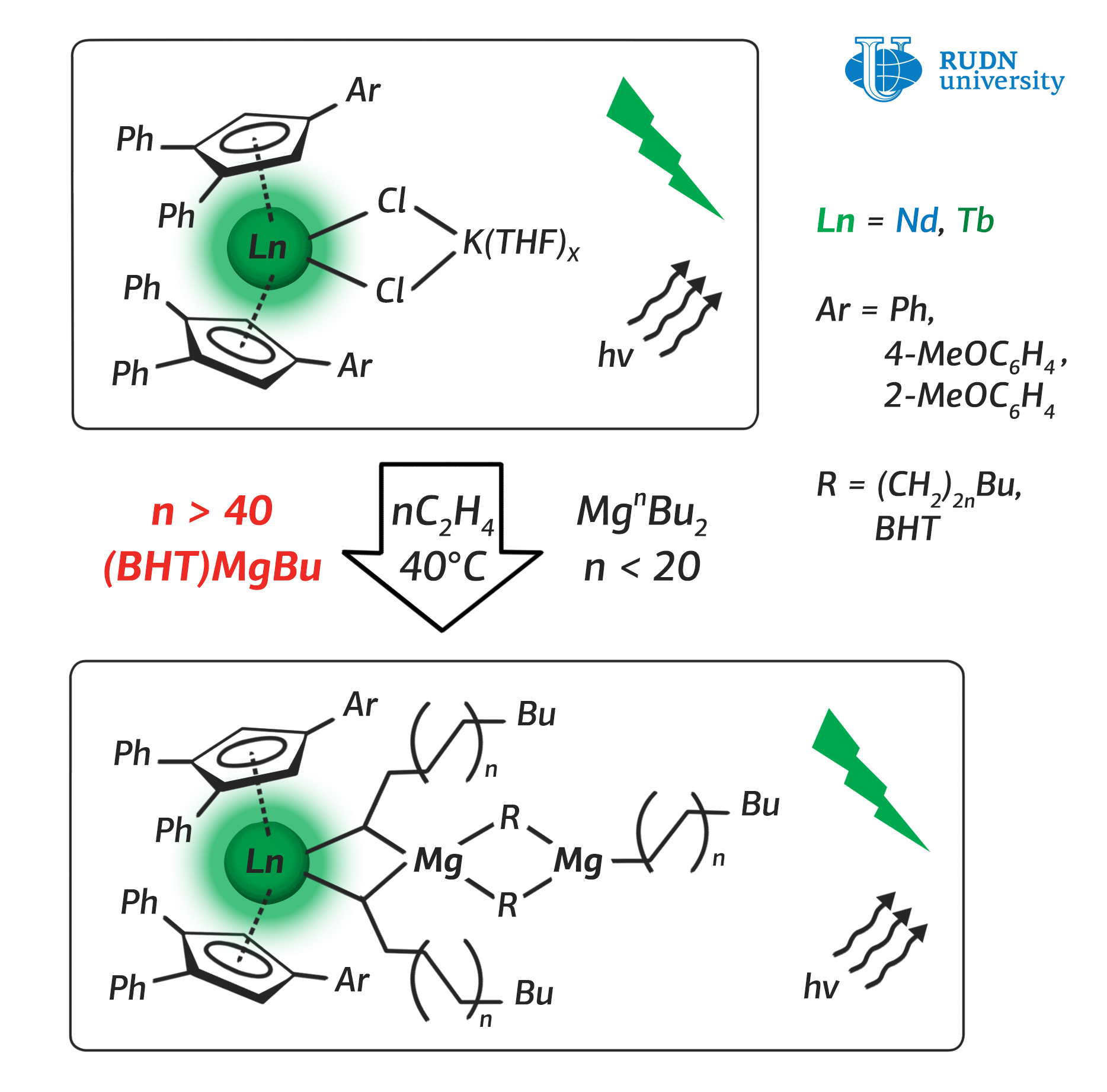
The chemists were able to synthesise three complexes of catalysts that included neodymium atoms. During the polymerisation reaction, they interacted with magnesium atoms, which is a part of the initiating agent of the reaction.
The scientists began experimenting with a widely used magnesium-based reaction initiating agent, which ensures the growth of ethylene chains at low (below 100 °C) temperatures. It consists of four alkyl groups, two for each magnesium atom. The use of this agent in the polymerisation reaction at a temperature of 40 °C allowed the chemists to obtain five chains of ethylene with a length of 16-20 molecules. By increasing the temperature of the solution to 80 °C, synthesists obtained ethylene chains of 70-150 molecules in length. However, these chains turned out to be unstable and have a tendency to split into short components.
So, the researchers came to the conclusion that to synthesise both long and stable ethylene chains it is necessary not to change the physical parameters (pressure and temperature) of the reaction, but to use a different reaction initiating agent, so that each alkyl group is bound to only one magnesium atom. They managed to create such an agent and start the polymerisation reaction with it. As a result of the experiment, the authors obtained three polyethylene chains up to 46 molecules long at 40 °C. The obtained samples of polyethylene were more flexible and durable compared to those that were created using current techniques.
All three catalysts, in combination with the new initiating agent the researchers created, gave similar results: polyethylene chains up to 46 molecules in length. It was confirmed not only by test results, but also by the molecular model developed by the authors of the paper.
The polyethylene sample synthesised by the new method turned out to be more durable and flexible in comparison with the polyethylene created on the basis of an ordinary reaction initiating agent. The technology has prospects for industrial applications in the production of plastic for food and bioprostheses.
The article was published in the journal Organometallics.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.