RUDN chemist, along with colleagues from the RAS Institutes, simplified the synthesis of antitumor compounds
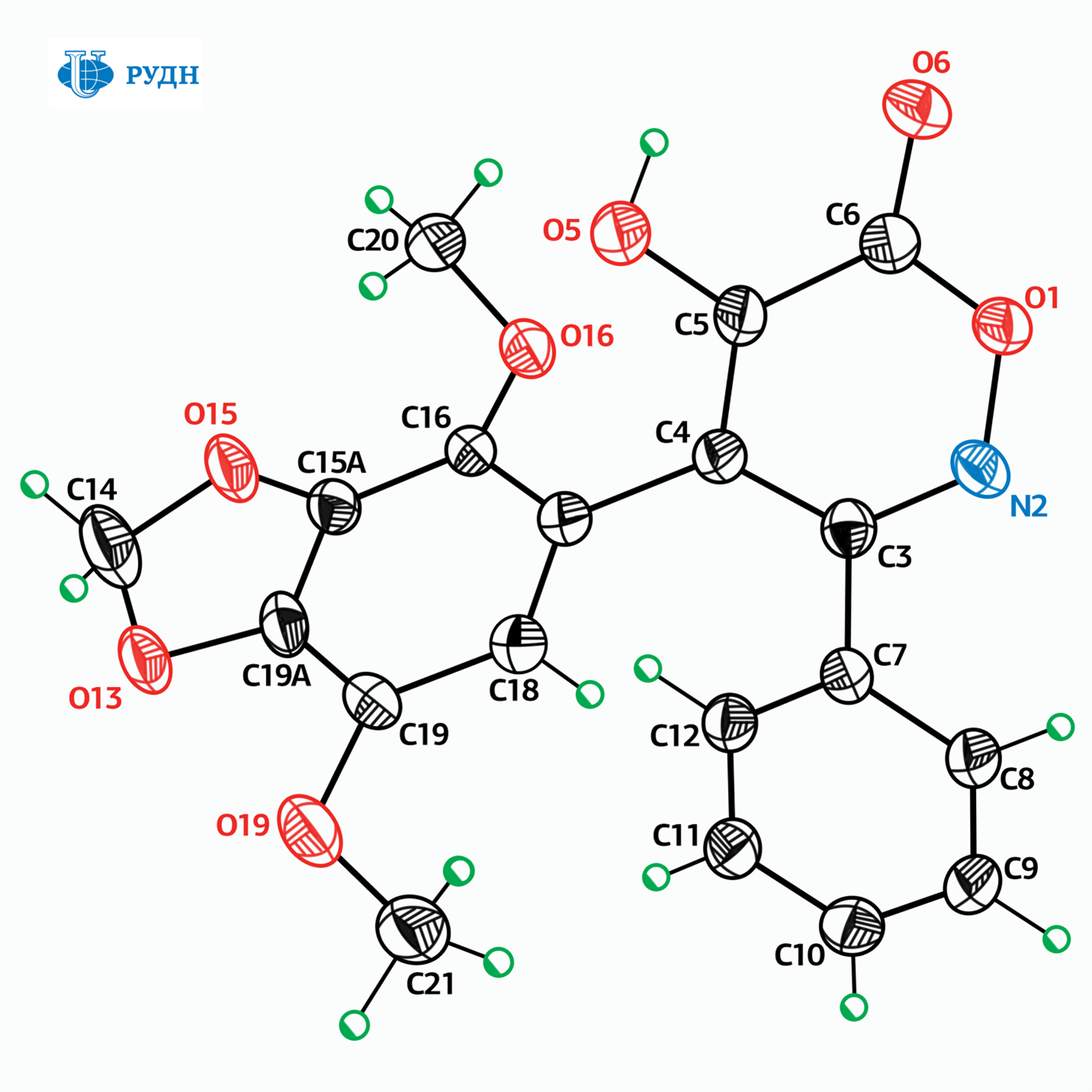
Many modern anticancer drugs are toxic, difficult to access and/or very expensive. Moreover, tumor cells may develop resistance to the drugs used. Therefore, researchers study the biological properties of molecules in order to obtain new antitumor drugs with optimal properties. One of the common approaches to the search for such drugs is testing of analogues of substances that already showed antitumor activity. Such substances include, in particular, isoxazole derivatives that inhibit — “turn off” — the Hsp90 protein, which is necessary for the survival of tumor cells. However, compounds of this class are inaccessible due to the complexity of the synthesis procedure, which requires, in particular, the complete absence of water molecules, and the reagents are expensive and toxic.
RUDN chemist Viktor Khrustalev and his colleagues developed a method for the synthesis of isomers of these substances, that is, the compounds that are identical in atomic composition but different in the arrangement of atoms in space. Easily accessible derivatives of arylnitromethanes and chloroacetamides were used as raw materials, and the reaction itself was carried out at temperatures not exceeding 80 degrees at atmospheric pressure and did not require anhydrous conditions.
The obtained substances had anticancer activity, but unlike the prototype compounds, they did not inhibit Hsp90 protein. Their mechanism of action is based on the destabilization of the cell division process as they prevent the formation of microtubules, which are important in the process of cell division.
Taxol derivatives, one of the most commonly used antitumor agents, have the same mechanism of action. Basing on the compounds obtained by the scientists, it is possible to create a substitute for an expensive, inaccessible and highly toxic derivatives of taxol in the treatment of cancer.
The work was published in European Journal of Organic Chemistry.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.