RUDN University chemist has found a way to improve solar cells
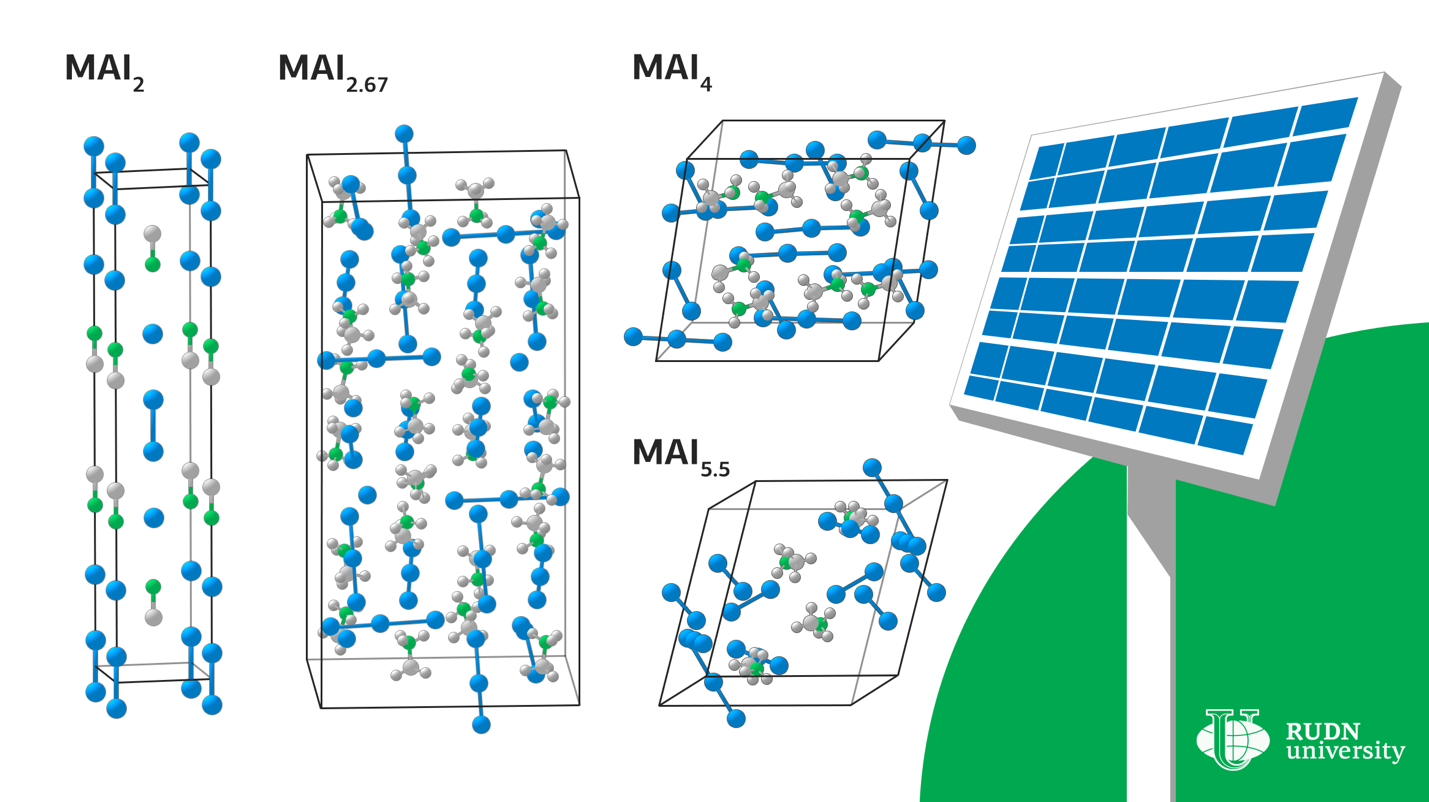
Lead-based hybrid perovskites are used in modern solar cells as a light-absorbing layer. But they are unstable to moisture, and existing technologies require the use of solutions and toxic solvents. This complicates the technology and makes it potentially dangerous. The solution to the problem can be solvent-free methods, that is, the use of melts rather than solutions — for example, you can apply a polyiodide melt to a thin film of metallic lead. However, there are few reliable studies of polyiodide chemistry. RUDN chemists investigated the properties of methylammonium (CH3NH3) and iodine compounds to find variants of compounds suitable for use in the production of perovskite solar cells.
Compounds of the methylammonium iodide (MAI) and iodine system melt at room temperature and form ionic liquids — melts that are composed exclusively of ions. The use of such liquids, which can be evenly applied to large surfaces, as precursors will bring closer the industrial production of modular solar cells based on hybrid perovskites on an industrial scale.
Already studied polyiodide-based liquids melt at room temperature only in the presence of large organic cations in the composition. RUDN chemist Viktor Khrustalev explained this difference by the fact that the methylammonium cation has a large dipole moment and is capable of forming a large number of hydrogen bonds. For small cation sizes, this leads to increased entropy during melting, which in turn lowers the melting point.
Under certain conditions, crystals of various compositions begin to fall out of the liquid-MAI2, MAI2.67, MAI4 and MAI5.5. In order to determine under what conditions the melt exists, scientists performed calculations of the enthalpy and entropy of the formation of these crystals. For all compounds except MAI2, the reaction of obtaining a compound from iodine and a composition with a lower iodine content proceeded solely due to the entropy contribution. RUDN chemists explained that the increase in enthalpy during the transition to compounds with a higher iodine content is due to the weakening of the interaction of cations with anions due to the distribution of a small negative charge on a large polyiodide anion. A similar increase in entropy is due to the complexity of polyanions and the weakening of the bonds between them.
These thermodynamic data made it possible to refine and generalize the experimental values of the boundaries in which the melt can exist.
Chemists have also found that similar effects occur in similar compounds with the formamidinium cation (FA+ = HC (NH2)2) and polybromides. Moreover, the mixed composition (MABr3) 0.15 (FAI3) 0.85 exhibits the properties of an ionic liquid from −40 to 80 °C. Such a low melting point of the precursor is favorable for the production of thin films of mixed hybrid perovskites, which exhibit maximum light-absorbing properties.
The article was published in Article in The Journal of Physical Chemistry Letters.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.