RUDN Chemist creates carbon “flower” for zinc-ion supercapacitors
Supercapacitors can store up to 100 times more energy, than conventional batteries, charge faster, and withstand more recharge cycles. One of the most promising supercapacitors is zinc — ion. However, its real capacities, which are still possible to achieve experimentally, are significantly less than those calculated theoretically. This is due to the limitations of the characteristics of carbon compounds that are used as cathodes. To find the best carbon structure, scientists are investigating carbon nanotubes, chemically activated graphene, layered porous carbon, and hollow уuranium spheres. A RUDN chemist has proposed a new 3D-structure that will improve the properties of zinc-ion supercapacitors.
“Hybridization-based supercapacitors have attracted considerable attention as promising platformsа for optimizing energy storage devices. However, limited by the insufficient quality of carbon cathodes, the energy capabilities of zinc-ion supercapacitors are inferior to those expected, especially at high power output,” - Raphael Luque, Center for Molecular Design and Synthesis of Innovative Compounds for Medicine, RUDN University.
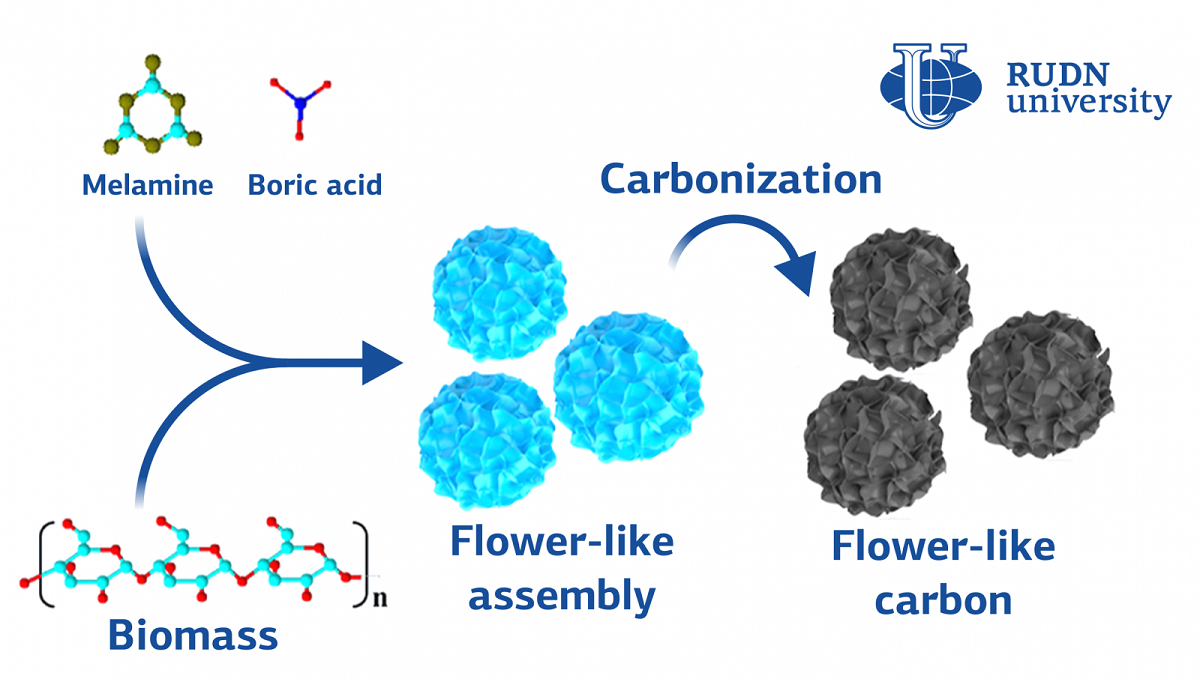
To get a new 3-D structure, chemists mixed melamine, boric acid and flour in water. The mixture was placed in an autoclave at 180 ° C for 15 hours. The result is structures similar in structure to carnation or hydrangea flowers — uneven balls with many pores. This “bouquet” chemists subjected to pyrolysis-for 2 hours heated, gradually increasing the temperature to 900 degrees. During pyrolysis, the auxiliary compounds in the “flowers” disintegrated, and only the carbon frame remained. Chemists performed similar procedures using flour and melamine, as well as only flour, as the starting compounds. All the resulting structures were studied using a scanning electron microscope. Then, using the resulting “flower” carbon (BCF), chemists made zinc-ion superconductors and measured its characteristics.
After comparing the structure of the obtained compounds, RUDN chemists concluded that boric acid did not affect the formation of the “flower” structure, and in fact оmelamine crystals and flour became the basis for it. It also turned out that BCF consists of many “nanolipes” — thin sheets connected to each other in a single ball structure. These bound nanolipes provided fast charge transfer within the flower and low resistance. The capacity of the BCF-based battery was larger than that of other similar devices — 133.5,5 mAh/ gram. The energy density (that is, the amount of energy that 1 kg of battery can store) also exceeded existing zinc-ion analogues.
“Suitable pores of the resulting carbon and the structure of its nanolipes ensure the penetration and exchange of electrolyte ions. Our research paves the way for creating carbon structures from individual carbon segments for energy storage devices,” - said Raphael Luque, Professor at the RUDN University Center for Molecular Design and Synthesis of Innovative Compounds for Medicine.
The results are published in the journal Carbon.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.