RUDN University chemists have created an unusual flat crystal with magnetic properties
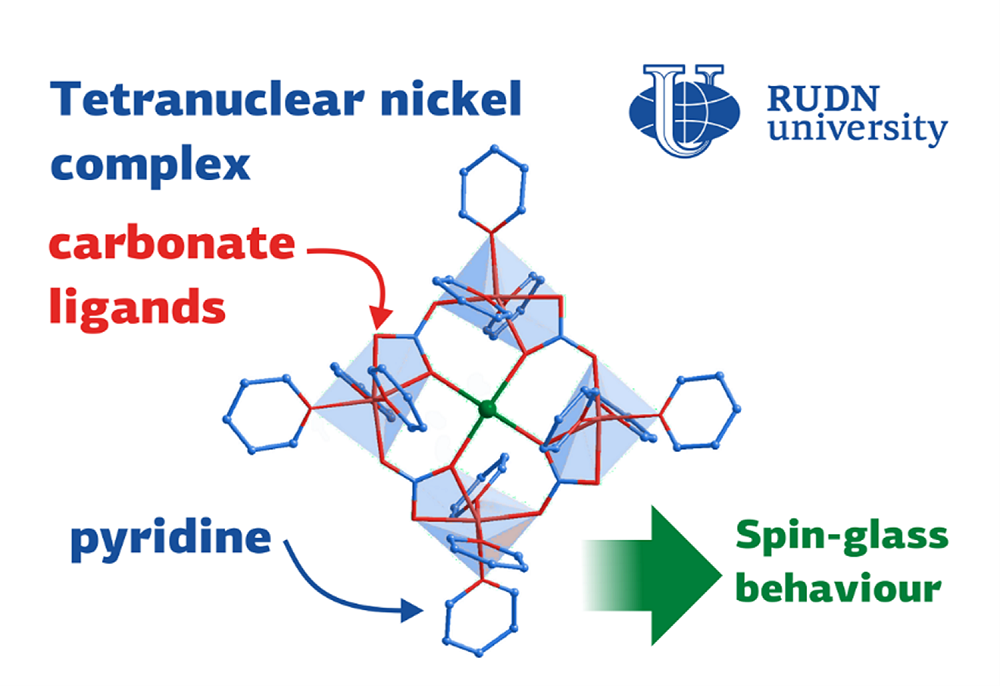
Coordination polymers are hybrid crystalline coordination compounds with a structure of infinitely repeating fragments (structural elements). The structure of the structural element includes metal centers and organic “bridges”. Coordination polymers are used for catalysis, separation of gas mixtures, creation of sensors, and storage of “guest” molecules. Some coordination polymers turn out to be molecular magnets with a linear chain structure, promising objects for creating high-capacity information storage devices. RUDN University chemists, studying the features of the synthesis of coordination polymers, created a new metal-containing compound with an unusual architecture, which turned out to be a molecular magnet (“spin glass”).
“The creation of molecular architectures based on transition metal ions using organic and inorganic ligands is attracting the attention of researchers due to its potential application in electronics, data storage, catalysis, and the creation of sensors and objects with luminescent properties,” Alexey Bilyachenko, Doctor of Chemistry, Leading Researcher at the Joint Institute chemical research of RUDN.
RUDN University chemists investigated the traditional protocol for creating coordination polymers using an organic compound with coordinating centers as binders. However, unusual organo-inorganic compounds (metallosilsesquioxanes) were used as the metal-containing center. The researchers used phenylsilsesquioxane containing nickel and sodium ions. At the last stage, chemists added pyridine, a colorless organic liquid with coordinating abilities, to the reaction mixture. As a result, a yellow crystalline product was isolated, the molecular structure of which was determined using X-ray diffraction studies of single crystals.
The substance that RUDN University chemists obtained turned out to be of unusual architecture. The complex has a flat structure resembling a square. The sodium cation is in the center of the square, the chloride anion, which equalizes the balance of charges in the complex, is located above the plane of the square. Four nickel ions forming a square structure are coordinated by pyridine ligands and additionally linked through carbonate bridges. The appearance of carbonate structural units (not used as reagents in the synthesis) is the most interesting observation in this reaction. Chemists suggested that the unusual carbonate bridges appeared due to the fact that during the reaction there was a spontaneous capture of carbon dioxide from the atmosphere. The carbonate fragments obtained in this way participate in the formation of the complex in a key way, forming the “sides” of the square. In this case, carbonates not only bind corner nickel ions, but also coordinate the central sodium ion. The chemists studied the magnetic properties of the crystals using a SQUID MPMS-XL magnetometer. It turned out that the new crystal is a molecular magnet exhibiting the properties of a spin glass.
“To our surprise, the reaction carried out causes a deep structural rearrangement with the formation of a structure with four nickel centers linked by carbonate bridges. The formation of such a compound cannot be explained by the formal logic of synthesis. Obviously, carbonates were formed as a result of the reaction of sodium ions with atmospheric CO2. The subsequent reaction of sodium bicarbonate with nickel ions resulted in the formation of the final molecular architecture. The arrangement of magnetically active nickel ions in the structure of a flat square provides an unusual magnetic behavior of the resulting complex, ”- Doctor of Chemistry Alexei Bilyachenko, Leading Researcher of the United Institute of Chemical Research of the Peoples’ Friendship University of Russia.
The results are published in the Journal of Organometallic Chemistry
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.