The chemist RUDN created a catalyst for more efficient oxidation of cyclohexane
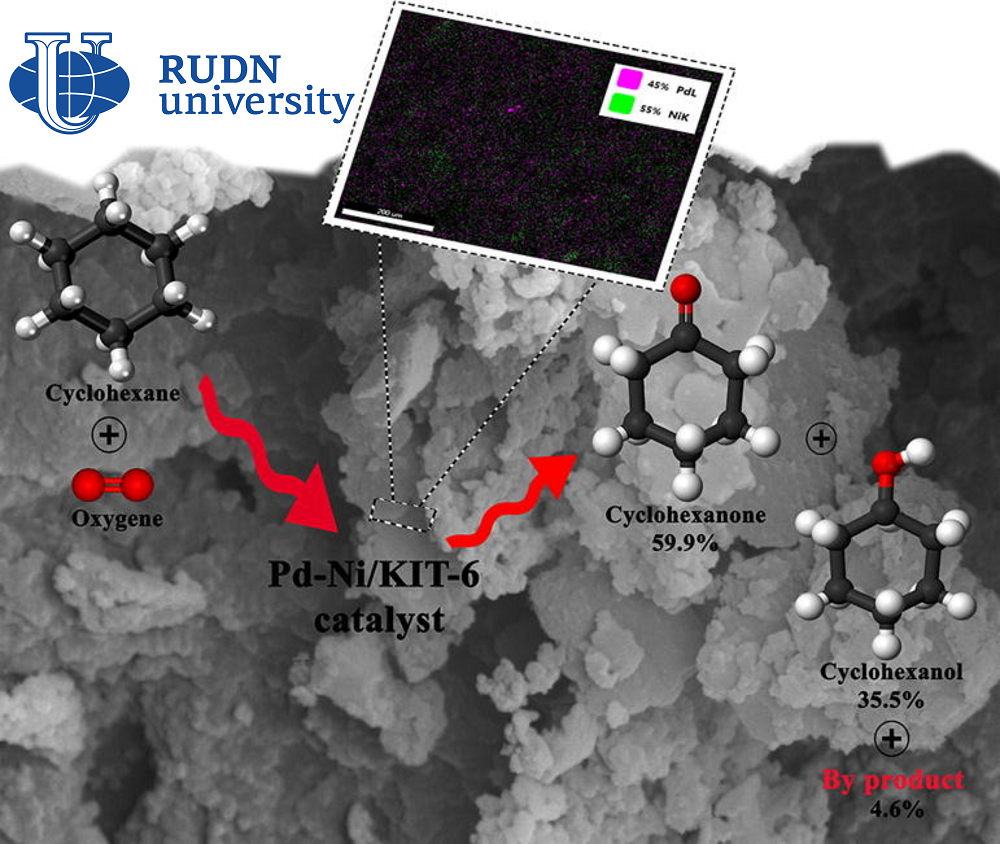
A mixture of cyclohesanol and cyclohesanone (these are two cyclic organic compounds that differ from each other by one hydrogen atom) is an important intermediate for the production of adipic acid. It is used for the production of household chemicals, building materials, food additives, polymers and other substances. In the production of cyclohexanol and cyclohesanone are obtained by oxidation of cyclohesan, and usually no more than 5% of the original cyclohesan is converted into the desired product. This is due to the fact that cyclohesanol and cyclohesanone have greater reactivity than their predecessor.
“The oxidation of cyclohexane to cyclohexanone and cyclohexanol is an important industrial process. These molecules are key intermediates in the production of caprolactam and adipic acid, which are used in the synthesis of nylon-6 and nylon-66 polymers. The industrial conversion of the matrix is usually less than 5%, since the target products are more active than the reagent,” — Rafael Luque, professor at the Center for Molecular Design and Synthesis of Innovative Compounds for Medicine of the RUDN.
The chemist RUDN together with colleagues from Iran and Spain created a catalyst 4.0%Pd-4.0%Ni/KIT-6 based on two metals — palladium and nickel, which are fixed on a porous structure of silicon dioxide. Chemists tested catalysts with different palladium and nickel contents, and also conducted a series of experiments to find optimal reaction conditions. The researchers then tested how many times the new catalyst could be used without loss of efficiency.
The most effective was a catalyst with 4% palladium and 4% nickel. The oxidation reaction of cyclohexane with such a catalyst takes place under the action of oxygen at 140 ℃ in an acetonitrile solution. In eight hours of reaction, 10.87% of cyclohexane is oxidized, and selectivity reaches 95.45%. This criterion is used to evaluate the effectiveness of the reaction and shows the ratio of the target product to all compounds obtained during the reaction. Under optimal conditions, only 4.55% of by-products are formed. With repeated use, the efficiency of the catalyst decreases, but not much. Over four cycles of use, the conversion rate (the amount of oxidized cyclohexane) dropped from 10.87% to about 8%.
“4.0%Pd-4.0%Ni/KIT-6 proved to be an effective catalyst for the oxidation of cyclohexane to cyclohexanol and cyclohexanone, showed excellent selectivity (95.45%) and good yield (10.37%) for the cyclohesanol-cyclohesanone mixture under optimal reaction conditions. The synergistic interaction of the two metals in the catalyst significantly increased both the yield of the final mixture and selectivity. Studies of the possibility of reuse of the new catalyst during four reaction cycles have shown that this material has retained its porous structure. This indicates excellent stability without a significant decrease in conversion values and selectivity after four cycles,” — Rafael Luque, professor at the Center for Molecular Design and Synthesis of Innovative Compounds for Medicine of the RUDN.
The results are published in the Journal of Industrial and Engineering Chemistry.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.