RUDN chemist obtained a reusable catalyst for the synthesis of esters
The catalysts are not consumed in the process of chemical reactions, yet it is difficult in some cases to separate them from the synthesis waste and reuse. For instance, inorganic acid catalysts are used for esterification, i.e. to obtain esters from organic acids and alcohol. In this case, the final product of reaction must be purified and the waste disposed of, along with the catalysts, since it is more expensive to separate them for reuse than to acquire new ones.
One promising solution is solid catalysts based on tin ions deposited on a porous support substrate. Its “active centres” are located on its surface: ions on which a chemical transformation occurs, for example, the formation of ether. However, tin ions are “washed out” during the use of such materials, and they lose their activity. Moreover, a lot of useless tin oxide is formed during the manufacture of the catalyst, in addition to ions.
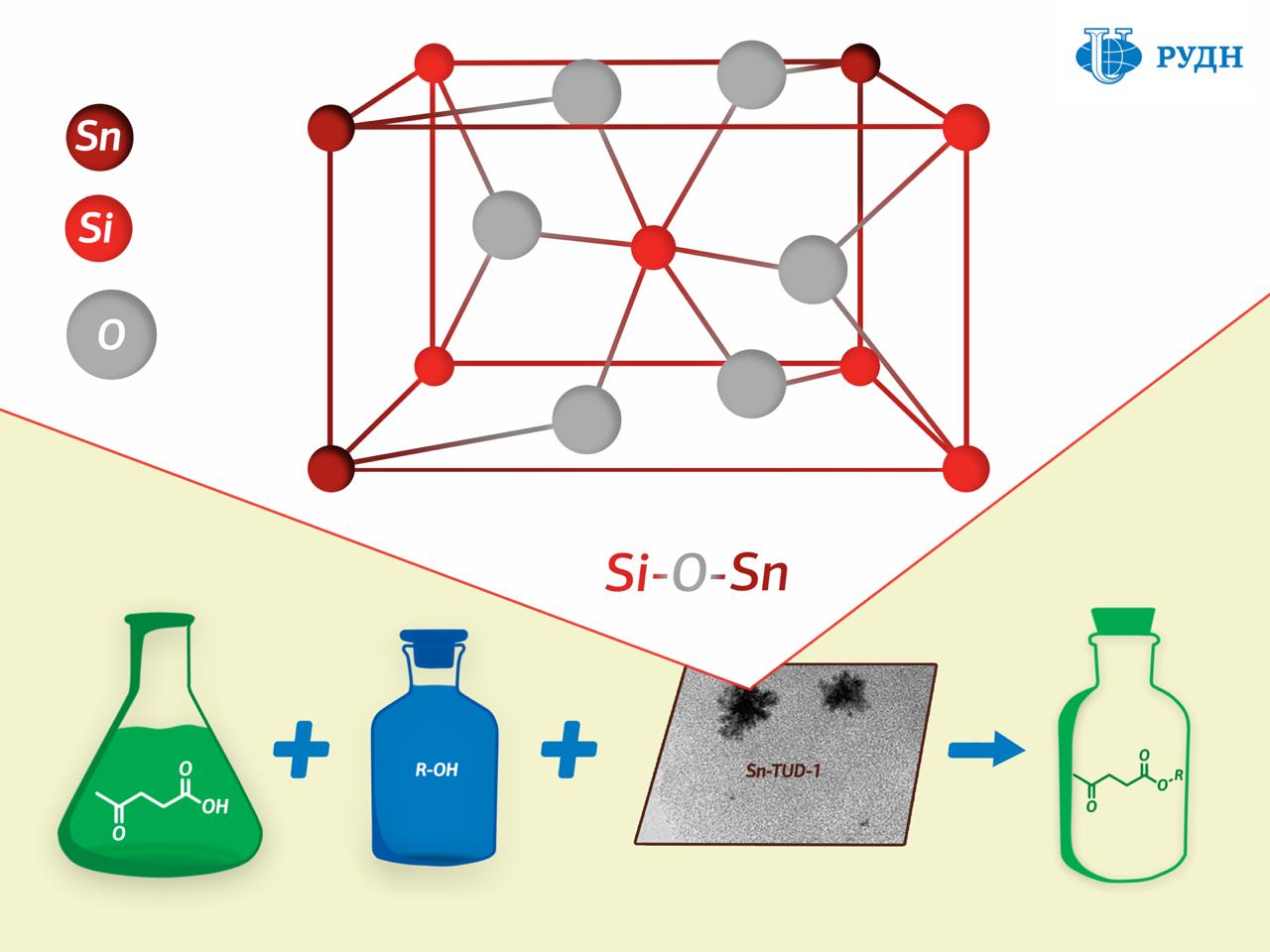
RUDN University chemist Rafael Luque has developed a new catalyst production method that results in a porous silicate matrix with “embedded” tin ions Sn4+ held together by strong chemical bonds.
While the existing methods for creating such catalysts involve tin being applied to a finished porous matrix of silicon dioxide, professor Luque formed the catalyst “from scratch”. The silicon dioxide substrate in his experiment was formed from a precursor (tetraethoxysilane) in the presence of tin, due to which tin ions were embedded in the chemical structure of the substrate.
The study of the substrate using XPS X-ray photoelectron spectroscopy showed that a chemical bond of silicon oxide and tin (Si–O–Sn) indeed formed in the catalyst.
The surface area of 1 gram of catalyst is significant — it’s 600 square metres. Since chemical reactions occur on the surface of a catalyst, the larger its surface area, the higher the activity. Most catalysts based on a silicon matrix have a useful area two to three times smaller: about 200-300 square metres per gram.
Chemists tested the activity of the new catalyst in the synthesis of levulinic acid esters. Levulinic acid is a product of the processing of carbohydrates such as glucose and starch. When interacting with alcohols it forms esters, which can be used as flavorings, plasticisers, and components of biofuels. It turned out that the new catalyst allows obtaining esters of levulinic acid with a maximum product yield of 44 to 99 percent - the figure corresponds to the efficiency of most commonly used catalysts.
In addition, the catalyst was tested for “reusability”: the experiment showed that its activity did not decrease after five regenerations.
The article in the journal Microporous and Mesoporous Materials
Products derived from microalgae represent a cutting-edge development in the field of bioeconomy. The potential of this biological resource was discussed at the international research seminar “Foundations for a Green Sustainable Energy”, part of the BRICS Network University’s thematic group on “Energy”. The event was organized by the Institute of Ecology at RUDN University.
Ambassadors of Russian education and science met at a conference in RUDN University to discuss how they can increase the visibility of Russian universities and research organizations in the world, and attract more international students in Russia.
The international scientific seminar hosted by RUDN Institute of Ecology “Experience of participation in student organizations as a way to form career skills” united scholarship recipients of the International Student Mobility Awards 2024 and Open Doors, along with members of the scientific student society “GreenLab” and the professional student association “Kostyor (Bonfire)” shared their projects focused on environmental protection.