RUDN University chemists proposed a way to reduce three times the temperature for the oxidation of alkanes
Until now, the reaction of catalytic oxidation of alkanes to alcohols, aldehydes, ketones, carboxylic acids could be carried out only at high temperatures — 150 degrees Celsius and above. Lowering the process temperature will simplify the synthesis and significantly reduce the cost of the final products. But this requires new catalysts.
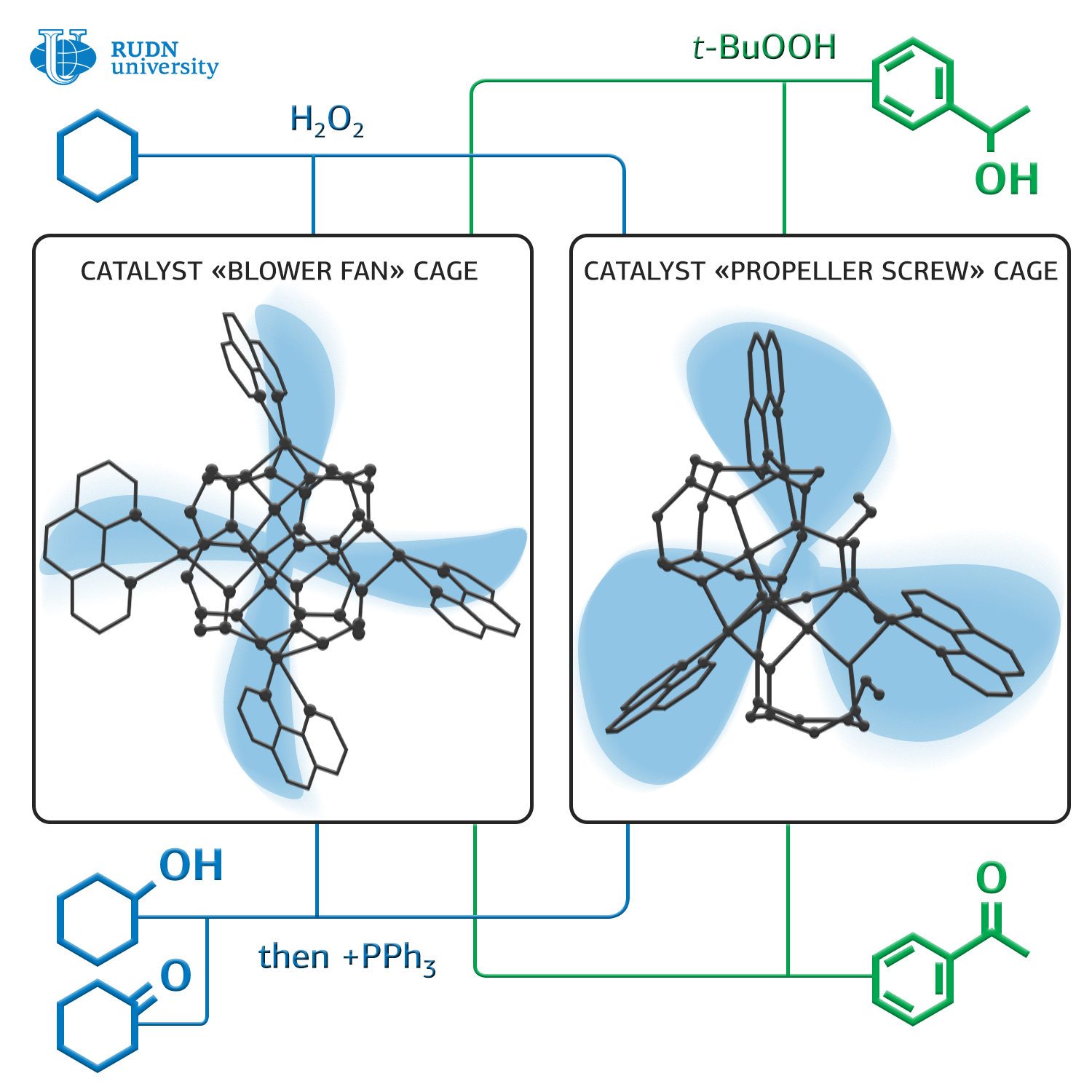
Chemists Georgy Shulpin and Alexey Bilyachenko from RUDN University together with colleagues from the Russian Academy of Sciences synthesized two new organoelemental compounds with a framework structure that can significantly reduce the oxidation temperature of alkanes — from 150 to 50 degrees Celsius.
These catalysts are based on silsesquioxanes — polymeric compounds with the general formula [RSiO3/2]n, (sesqui in Latin means “one and a half”). For their synthesis, a simple two-stage scheme is used. The first structure is formed in tetrahydrofurane and contains Cu4Na4, the second in dimethylformamide and contains Cu5. RUDN University chemists studied the molecular structure of the obtained compounds, and also a structure of the supramolecular structures formed by them in crystals.
The researchers tested the catalytic activity of these compounds using them as catalysts for the oxidation reaction of hexalnethylene to cyclohexanol and cyclohexanone under the action of hydrogen peroxide in acetonitrile at 50 degrees. The conversion — the ratio of the amount of the obtained product to the theoretically possible amount — was about 25% in this reaction, which is comparable to the indicators of the traditional high-temperature method. In addition, chemists used these catalysts in the oxidation reaction of cyclohexanol to cyclohexanone and 1-phenylethanol to acetophenone under the action of tert-butyl hydroperoxide at the same temperature. The conversion in the case of cyclohexanol was about 40%, and the oxidation of 1-phenylethanol to acetophenone was almost complete. Thus, chemists took an important step to simplify the technology of the synthesis of a number of important compounds for industry.
Chemists emphasize that the production of cyclohexanone from hexalnethylene is important since from cyclohexanone adipic acid is obtained — the raw material for the production of nylon-6,6, and caprolactam — the raw material for the production of capron (nylon-6). Now in the industry, the process of synthesis of cyclohexanone from hexalnethylene is carried out at a temperature of about 150 degrees; the new method will reduce the temperature to 50 degrees.
The article is published in the journal Catalysts.
Products derived from microalgae represent a cutting-edge development in the field of bioeconomy. The potential of this biological resource was discussed at the international research seminar “Foundations for a Green Sustainable Energy”, part of the BRICS Network University’s thematic group on “Energy”. The event was organized by the Institute of Ecology at RUDN University.
Ambassadors of Russian education and science met at a conference in RUDN University to discuss how they can increase the visibility of Russian universities and research organizations in the world, and attract more international students in Russia.
The international scientific seminar hosted by RUDN Institute of Ecology “Experience of participation in student organizations as a way to form career skills” united scholarship recipients of the International Student Mobility Awards 2024 and Open Doors, along with members of the scientific student society “GreenLab” and the professional student association “Kostyor (Bonfire)” shared their projects focused on environmental protection.