Scientists report an effective method for synthesizing analogues of natural medicines
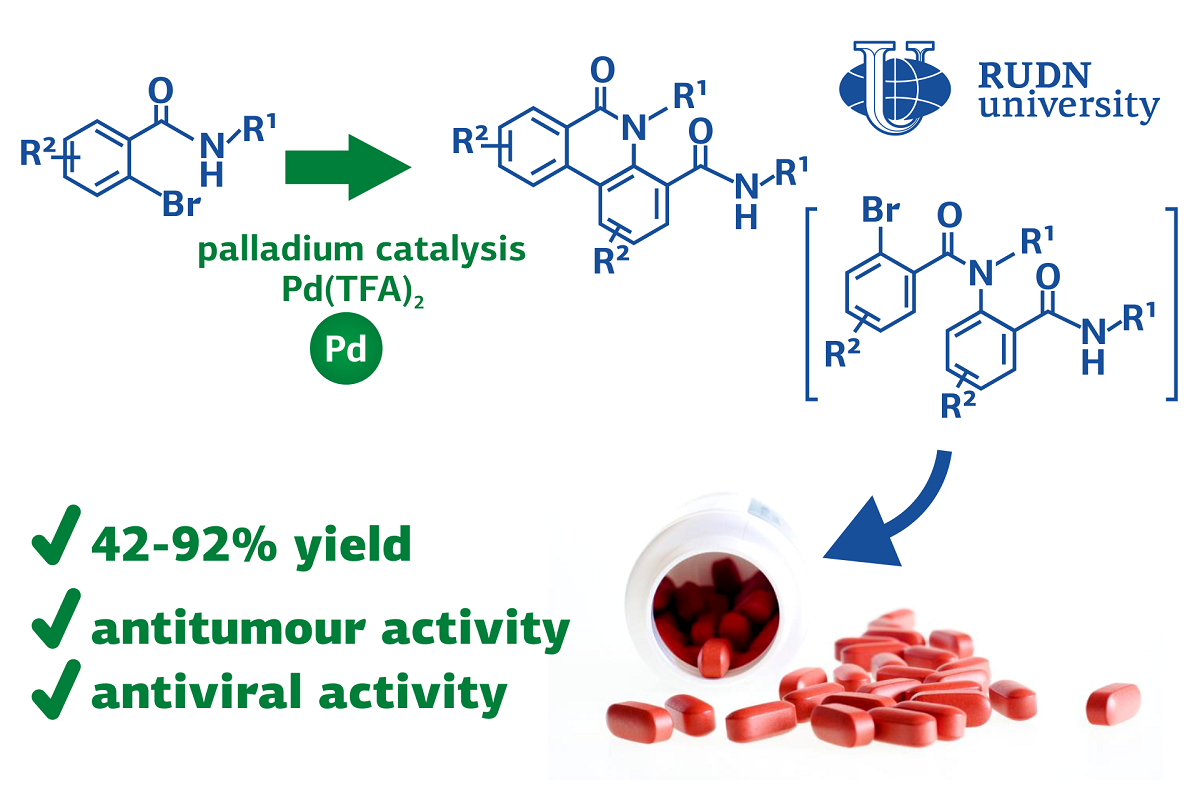
Organic compounds phenanthridinones are part of many natural substances with medicinal activity, including antiviral and antitumor. There are several strategies for creating them in the laboratory, but all of them are either time-consuming or require harsh conditions. Most methods of synthesis are essentially limited since they involve the connection of two ring fragments (aryls). The RUDN University chemist, together with colleagues from Belgium and China, proposed a different approach, to combine not pure aryl fragments, but modified ones — with the nitrogen atom.
“Conventional strategies involve multistep procedures or harsh reaction conditions. However, these reaction systems are always limited to firstly suffer from aryl-aryl coupling, preventing the design of new and efficient strategies to synthesize diverse and complex phenanthridinones. Based on this situation, we set out to exploit whether a pathway firstly undergoing N-arylation instead of aryl-aryl coupling would be possible for the synthesis of phenanthridinones.,” said Erik Van der Eycken, the head of the Joint Institute for Chemical Research at RUDN University.
Chemists suggested a palladium-based catalyst. After testing 17 types of palladium catalysts, scientists found that the most effective compound was palladium and trifluoroacetic acid. The best auxiliary substances were potassium carbonate (it served as a base), and the best solvent was dioxane.
Having determined the optimal conditions, the chemists conducted a series of experiments with different starting compounds — 25 types of bromobenzamides. The reaction takes 12 hours at a temperature of 100 ° C and gives different phenanthridinones at the output with an efficiency of 42-92%. The resulting products can still be diversified and more complex phenanthridinones can be obtained with a yield of 30-75%.
“We have developed a novel method to prepare diverse phenanthridinones from bromobenzamides through palladium-catalyzed cascade intermolecular N-arylation/aryl-aryl coupling process. This reaction features excellent chemo- and regioselectivity, broad substrate scope, excellent functional group tolerance and moderate to excellent yield. The synthetic utility of this method is successfully illustrated by the further late-stage diversification of the obtained phenanthridinones. This method also provides a new direction for the synthesis of diverse and complex phenanthridinones.,” said Erik Van der Eycken, the head of the Joint Institute for Chemical Research at RUDN University.
The results are published in Molecular Catalysis.
Products derived from microalgae represent a cutting-edge development in the field of bioeconomy. The potential of this biological resource was discussed at the international research seminar “Foundations for a Green Sustainable Energy”, part of the BRICS Network University’s thematic group on “Energy”. The event was organized by the Institute of Ecology at RUDN University.
Ambassadors of Russian education and science met at a conference in RUDN University to discuss how they can increase the visibility of Russian universities and research organizations in the world, and attract more international students in Russia.
The international scientific seminar hosted by RUDN Institute of Ecology “Experience of participation in student organizations as a way to form career skills” united scholarship recipients of the International Student Mobility Awards 2024 and Open Doors, along with members of the scientific student society “GreenLab” and the professional student association “Kostyor (Bonfire)” shared their projects focused on environmental protection.