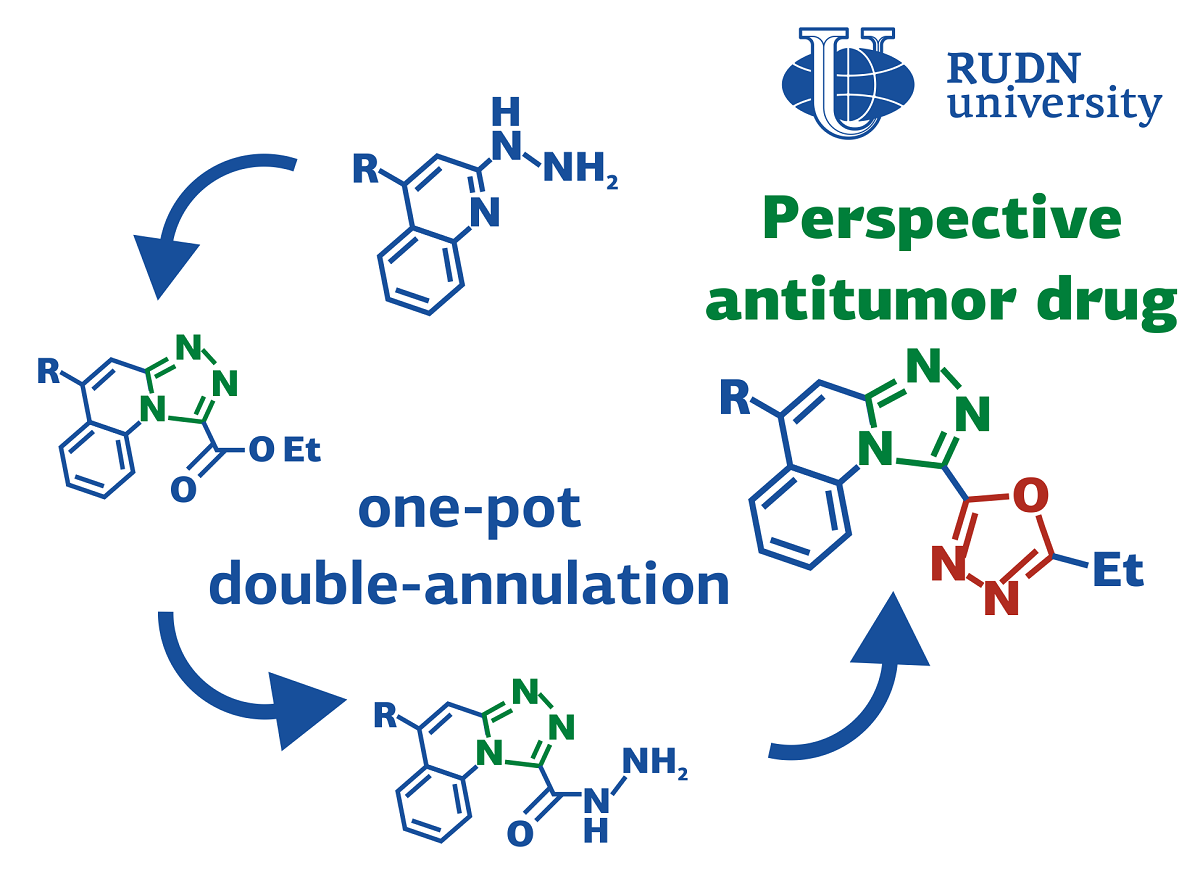
Simple protocol for the potential anti-cancer drugs synthesis
Derivatives of organic substances quinolines are used in pharmaceuticals to develop potential antihypertensive, anticonvulsant and anti-inflammatory drugs. Their biological activity can be enhanced by adding cyclic fragments to their structure. For example, the complex of the cyclic compound oxadiazole and a quinoline derivative has potential anticancer activity. To obtain other similar compounds and test their activity, it is necessary to find an effective way to synthesize them. Such a protocol was proposed by a team of chemists from the North Caucasus Federal University, RUDN University and the University of Kansas (USA) under the guidance of Doctor Alexander Aksenov.
“Recently, we described the preparation of novel antitumor agents with in vitro differentiation activity against neuroblastoma cancer cell lines. we had a task of building a focused library of perspective ‘chimeric’ antitumor drug candidates possessing both triazoloquinoline and oxadiazole rings. An expeditious and concise synthetic method was needed to allow for a highly efficient installation of both heterocyclic cores in a single-pot fashion”, said Elena Sorokina, PhD, associate research professor at the Department of Organic Chemistry of the RUDN University.
The key to the synthesis of compounds with different cyclic components were nitroalkanes — the derivatives of alkanes with added nitrogen complex. They act like electrophiles meaning that they can accept an electron pair and form a chemical bond. This gives the opportunityy for a “one pot” type reaction, when all the reagents are mixed in a single reaction vessel and come together without additional intervention.
Chemists have tested this synthesis approach experimentally. Scientists have selected optimal conditions — temperature, reagents and a medium. As a result, at a temperature of 130℃ in polyphosphoric acid, 90% of the reaction efficiency was achieved (the experimental yield of the product was 90% of the calculated theoretically). To test the universality of the method, chemists obtained more than 10 compounds with a yield of 55%-90% according to this principle. Scientists have suggested that this method can also be used for the synthesis of more complex substances, in which even more cyclic fragments are combined.
“The method provides an expeditious and direct access to triazoloquinolines bearing an oxadiazole substituent, which might be of interest for medicinal chemistry. Furthermore, this research paves the road for development of other acid-mediated cascade transformations for preparation of complex heterocyclic compounds. In principle, the same strategy can potentially be employed for stepwise assembly of longer linear oligomeric chains with repeating oxadiazole units, provided that greater annulation efficiency could be achieved at every step. Synthesis of a focused library for biological studies is currently underway in our laboratories”, said Elena Sorokina, PhD, associate research professor at the Department of Organic Chemistry of the RUDN University.
The results are published in Molecules.
Products derived from microalgae represent a cutting-edge development in the field of bioeconomy. The potential of this biological resource was discussed at the international research seminar “Foundations for a Green Sustainable Energy”, part of the BRICS Network University’s thematic group on “Energy”. The event was organized by the Institute of Ecology at RUDN University.
Ambassadors of Russian education and science met at a conference in RUDN University to discuss how they can increase the visibility of Russian universities and research organizations in the world, and attract more international students in Russia.
The international scientific seminar hosted by RUDN Institute of Ecology “Experience of participation in student organizations as a way to form career skills” united scholarship recipients of the International Student Mobility Awards 2024 and Open Doors, along with members of the scientific student society “GreenLab” and the professional student association “Kostyor (Bonfire)” shared their projects focused on environmental protection.