RUDN University biologist found that Chinese date improves the immune system of fish
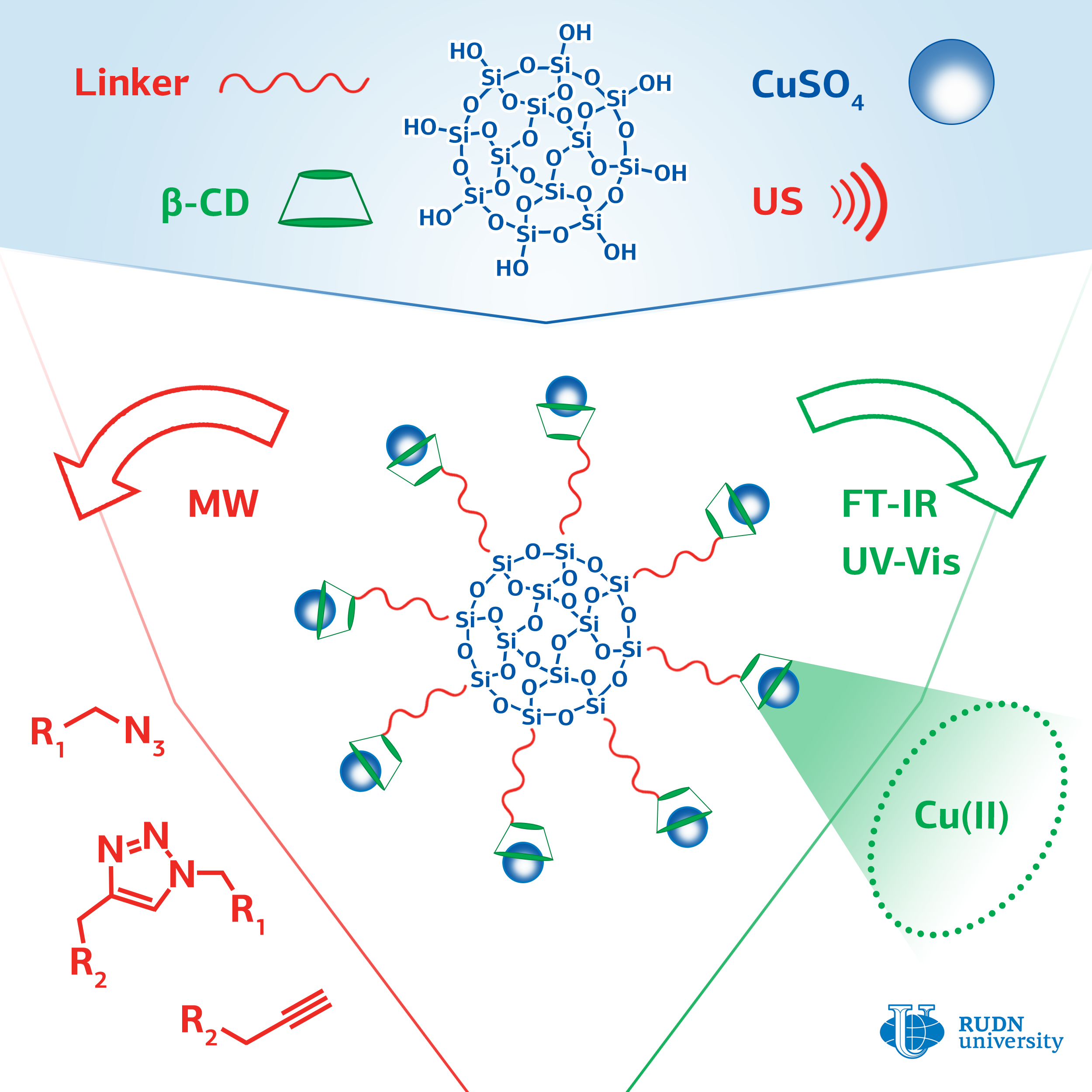
Click chemistry methods are used to synthesize libraries of substances with high chemical diversity, which is important when developing new drugs. These reactions are necessary for introduction of labels (for example, fluorescent ones) into biological macromolecules, proteins, and DNA molecules. This is used in biological and medical research.
The most commonly used click chemistry reaction is the addition of a substance that contains a carbon-carbon triple bond (alkine) to a compound containing a fragment with three nitrogen atoms in a row (azide). The classic version of the reaction involves the use of copper in oxidation state (I) as a catalyst. For this, ions of copper (II) and an excess of a reducing agent are introduced into the reaction, or copper (I) is used and the reaction is conducted with protection against oxygen, which imposes certain restrictions on the application of this reaction.
A chemist from RUDN University Rafael Luque and his colleagues have developed a series of catalysts with copper ions attached to the surface of silica gel particles using cyclic cyclodextrin oligosaccharide. Cyclodextrin consists of seven glucose molecules closed in a cycle. Inside the cycle there is a container that can hold the copper ion and increase its catalytic activity. Ultrasound irradiation was used to facilitate the binding of cyclodextrin to the surface of silica gel.
The effectiveness of the created catalysts was evaluated on a model reaction of phenylacetylene with benzylazide. The researchers managed to achieve a yield of the reaction product of more than 99%. The yield with copper (II) acetate was 14%, and in the case of copper (II) sulfate, the reaction did not occur at all. The method for producing the catalyst is simple, safe for the environment, and cheap; its use does not require to add reducing agents or oxygen-free conditions. The catalysts can find application in the pharmaceutical industry and in biomedical research.
The paper was published in the journal Molecules.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.