RUDN University chemists developed cheap and eco-friendly surfactants
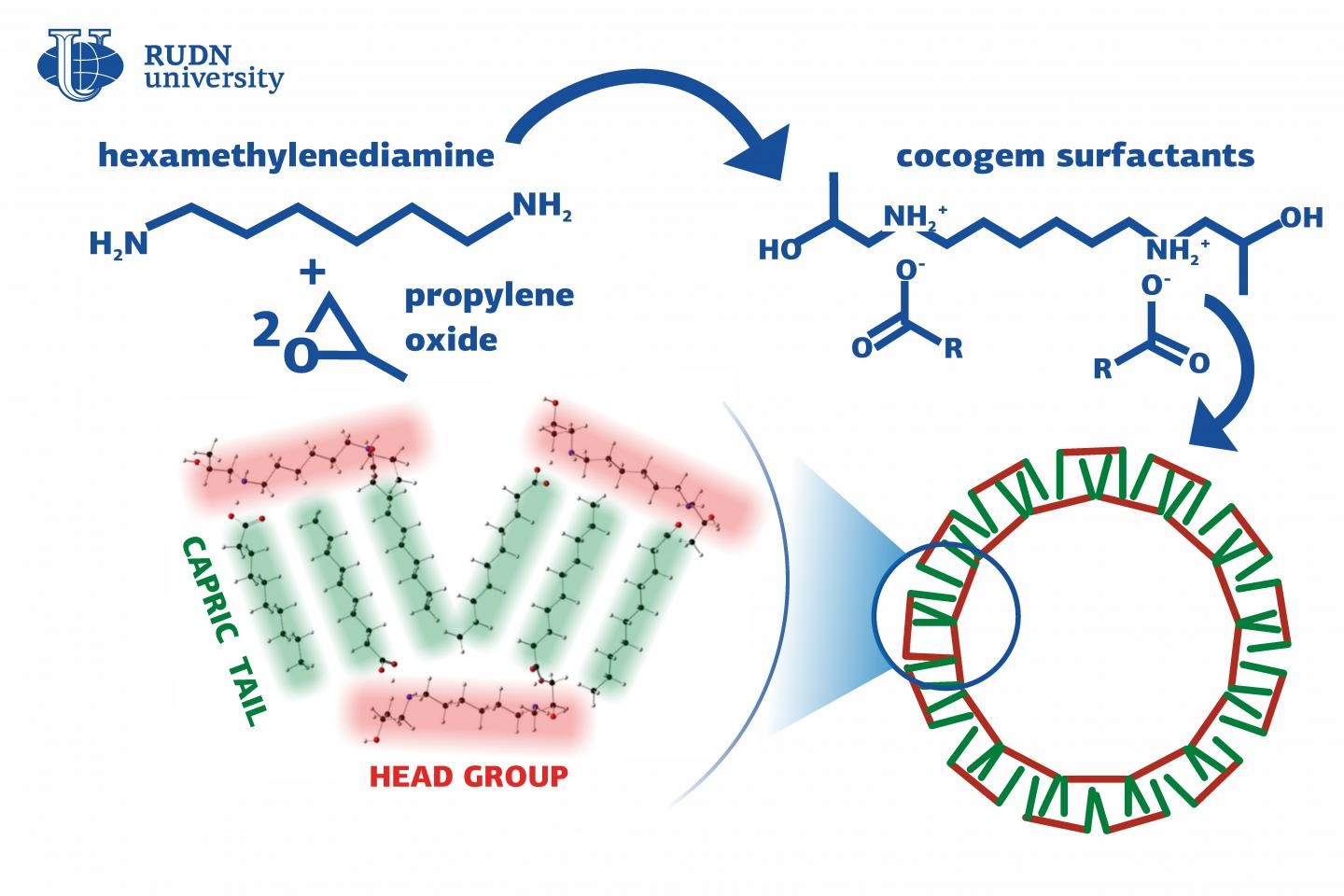
Surfactants are common both in the industry and in our everyday life. They are used as bases for detergents, added to lubricants and construction materials (concrete mixes, cement, and drilling fluids), and protect equipment from corrosion. The most effective of them are gemini surfactants that contain two hydrophilic (water-attracting) groups. However, there also “pseudo” gemini surfactants that have one hydrophilic and one hydrophobic (water-resistant) group of atoms bound together. An international team of chemists including partners from RUDN University synthesized several new “pseudo” gemini surfactants and confirmed that their performance characteristics were on par with the existing compounds. The new synthesis method is quite cost-efficient, works at room temperature, and does not require expensive reagents.
“The creation of cheap methods for the synthesis of gemini surfactants with satisfactory performance characteristics can be a turning point for the chemistry of detergents. We focused both on the development of synthetic methods and on studying the properties of new compounds,” said Fedor Zubkov, a PhD, and an Assistant Professor at the Department of Organic Chemistry, RUDN University.
To create new compounds, the chemists used six common higher fatty acids: capric, lauric, myristic, palmitic, stearic, and oleic acid. The hydrophilic properties of the new surfactants came from amines synthesized based on two affordable reagents—hexamethylenediamine and epoxypropene. To obtain a surfactant, the team dissolved an amine and one one the acids (at the ratio 1:2) in acetone at room temperature and then concentrated the solvent. The final products looked like viscous yellow liquids. The molecules of the surfactants were formed due to electrostatic interaction between oxygen atoms in the carboxylic groups of the acids and amino groups in the amine.
The team at RUDN University conducted a thorough study of the properties of the new compounds and paid special attention to the key characteristic of surfactants—their ability to reduce the superficial tension of solutions and form micelles. It is this property that makes surfactants usable in the skincare industry. The lowest superficial tension values (up to 20 mN/m) were registered for the surfactant based on myristic acid. By this parameter, the new surfactant is almost identical to fluorine-containing ones that are often used in the industry. However, unlike them, the new compound is more environmentally friendly. Weaker chemical bonds make it biodegradable and therefore unlikely to be accumulated in the environment.
Other important characteristics of surfactants are foam formation and foam stability. Five out of six new surfactants formed foam in a water solution, and in the case of two of them (based on lauric and myristic acids), the volume of produced foam exceded the volume of the initial solution 3 to 4 times. The compounds based on palmitic and stearic acids produced less foam, but it was more stable and lasted up to three days. These and other characteristics of the new compounds make them promising for the skincare industry, medicine, anti-corrosion treatment of construction and industrial facilities, and oil recovery increase.
“Gemini surfactants and their synthesis are a burning issue for modern chemistry, as they can potentially replace traditional monomeric surfactants and make the chemical industry move environmentally friendly,” added Fedor Zubkov.
The article was published in Journal of Molecular Liquids.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.