A chemist from RUDN University synthesized analogs of natural toxins
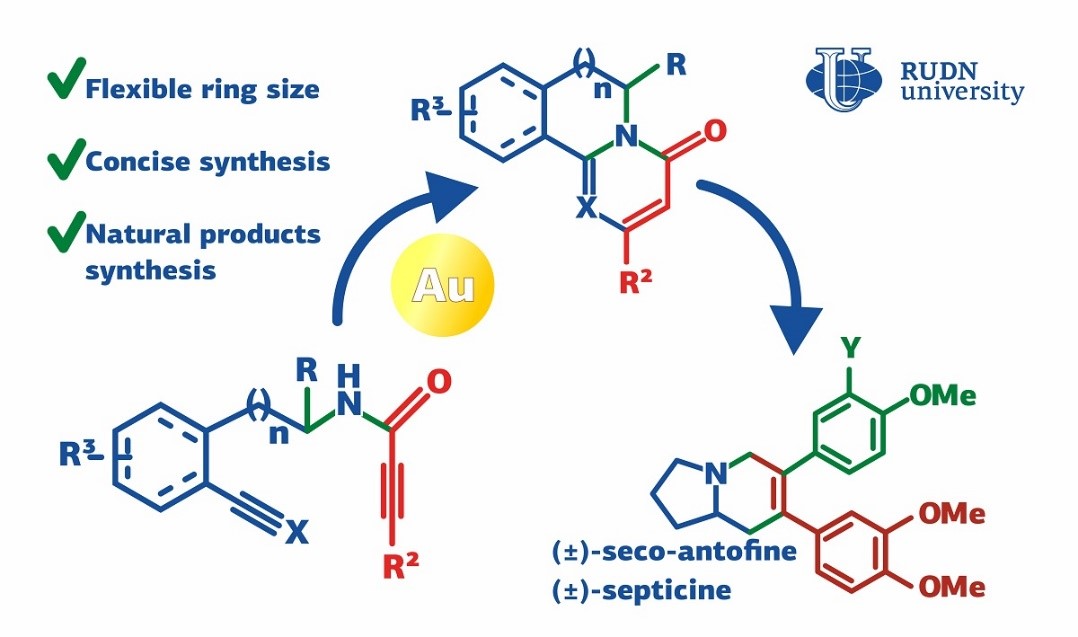
Antofine and septicine have antibacterial and antitumor properties and therefore can be used in the pharmaceutical industry. However, it is difficult to obtain them directly from natural plant sources: they are hydrophobe and can sometimes be unexpectedly toxic. A chemist from RUDN University developed a universal method to produce 2-pyridone derivatives on which antofine and septicine are based.
2-pyridone and 4-pyrimidone are cyclic molecules with one oxygen atom attached to the cycle. The cycle of the former compound contains one nitrogen atom, and of the latter--two. Both substances are used as molecular frameworks for medicinal drugs. By attaching additional cycles and atoms to them, one can obtain compounds with antitumor, antiviral, anti-inflammatory, and antimalarial properties. The developed method of 2-pyridone and 4-pyrimidone production consists of just 2 steps.
The first step is the so-called four-component Ugi reaction. Using it, one can obtain peptide fragments (analogs of proteins) from four simple substances. In his experiment, the chemist from RUDN University used different substances at room temperature and the Ugi reaction went on for 12 hours. As a result, around 60 different compounds were obtained, and in all of them, a hydrocarbon ring was attached to other groups of atoms with peptide bonds.
In the next step, several new cycles had to be created in the peptide fragment, and at least one of them had to contain nitrogen atoms. To achieve this, the chemist suggested using gold-based catalysts. Out of five tested catalysts, one produced the best 2-pyridone yield (75%). This result was achieved in the reaction with the first of the synthesized peptide fragments. Further studies showed that the use of other first-stage products can lead up to 93% yield of the products with the required molecular frameworks. The same approach was used for the synthesis of 4-pyrimidone derivatives.
"The main advantage of our method is the ability to develop organic substances based on heterocycles with different functional groups. This advantage appears at the stage of the four-component Ugi reaction, as many simple and affordable reagents can be used in it. The cyclization reaction suggested by us can involve reagents with different functional groups. The simplicity of this approach and a wide range of potential results make it favorable for medicinal chemistry," said Erik Van der Eycken, the head of the Joint Institute for Chemical Research at RUDN University.
The research was published in the Organic Letters journal.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.