RUDN Chemists discovered three complex compounds with rare magnetic properties
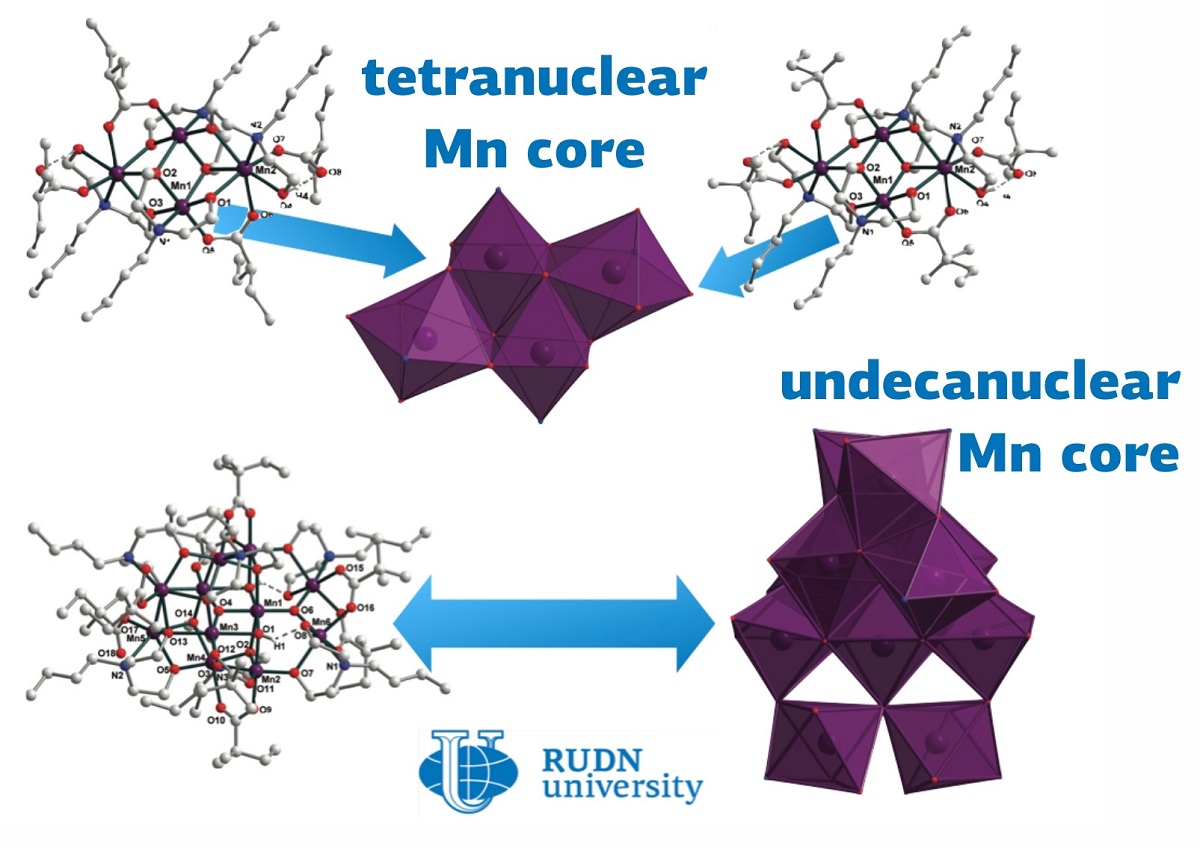
Coordination complex is complex structure with a metal atom in the center, to which various ligand molecules are attached. If there are more than two of these atoms, the compounds are referred to as multinuclear. In this case, the metal framework can look like chains, branched cycles, polyhedra or their combinations. Non-standard bonds between metals allow obtaining compounds with unusual metal oxidation states and pronounced catalytic properties. This allows their application in the synthesis of drugs, varnishes, and paints, as well as in other branches of the chemical industry. In addition, the magnetic properties of such complexes can be used for new ways to store information. Manganese also forms complex compounds, for example, inside chlorophyll, through which photosynthesis occurs. However, multinuclear complexes with manganese are often unstable. RUDN chemists reported the synthesis of several compounds of this class at once.
"In this paper, we describe the synthesis methods, crystal structure, and magnetic properties of three new multinuclear mixed valence clusters that we were able to obtain from manganese (II) chloride," co-author Dmitro Nesterov from RUDN.
The valence is indicated by Roman numerals and shows the ability to form a certain number of chemical bonds. Two of the described compounds are tetranuclear and contain two manganese atoms each with valence II and two each with valence III. In [MnII2MnIII2(HBuDea)2 2(BuDea)2(EBA)4] the ligands were 2-ethyl ether of butyric acid and N-butyl diethanolamine, and in the second compound [MnII2MnIII2(HBuDea) 2(BuDea)2(DMBA)4] - N-butyl diethanolamine and 2,2-dimethyl ether of butyric acid. In the third compound, three manganese II atoms, eight manganese III atoms, and four oxygen atoms form an eleven-nuclear structure to which the ligands N-butyldietanolamine and 2,2-dimethyl ester of butyric acid are attached.
RUDN chemist and his colleagues from Slovakia and Portugal succeeded in obtaining these complexes using self-assembly reactions. The synthesis requires manganese (II) chloride, a solution of carbolic acid in methanol, and 2-ethyl ester of butyric acid for the first compound, and 2,2-dimethyl ester of butyric acid for the second and third. Whether the second route produced a four-nuclear or an eleven-nuclear cluster depended on the experimental conditions. X-ray crystallography showed that both quadruple nuclei had a similar symmetrical structure, while the third had a non-standard structure. The quaternary complexes exhibited the properties of a single-molecule magnet - that is, they can form superparamagnetic materials. This means that they can be uniformly magnetized throughout their volume and change their magnetic moment depending on temperature. The eleven-nucleus cluster, on the contrary, had antiferromagnetic properties, i.e., the magnetic moments of the particles in such a substance are in pairs directed in opposite directions.
"Also in the paper we discussed the possible influence of intramolecular effects and the different surroundings of the magnetic nuclei that the 2-ethyl ester of butyric acid and 2,2-dimethyl ester of butyric acid ligands formed. Superparamagnetics and antiferromagnetics exhibit unusual properties that could be used in future high-tech applications. For example, they can become the basis for new generation memory cells, where only a few tens of atoms are required to record information," Nesterov added.
The results of the study were published in Dalton Transactions.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.