RUDN University chemist proposed a copper catalyst for the synthesis of biologically active substances
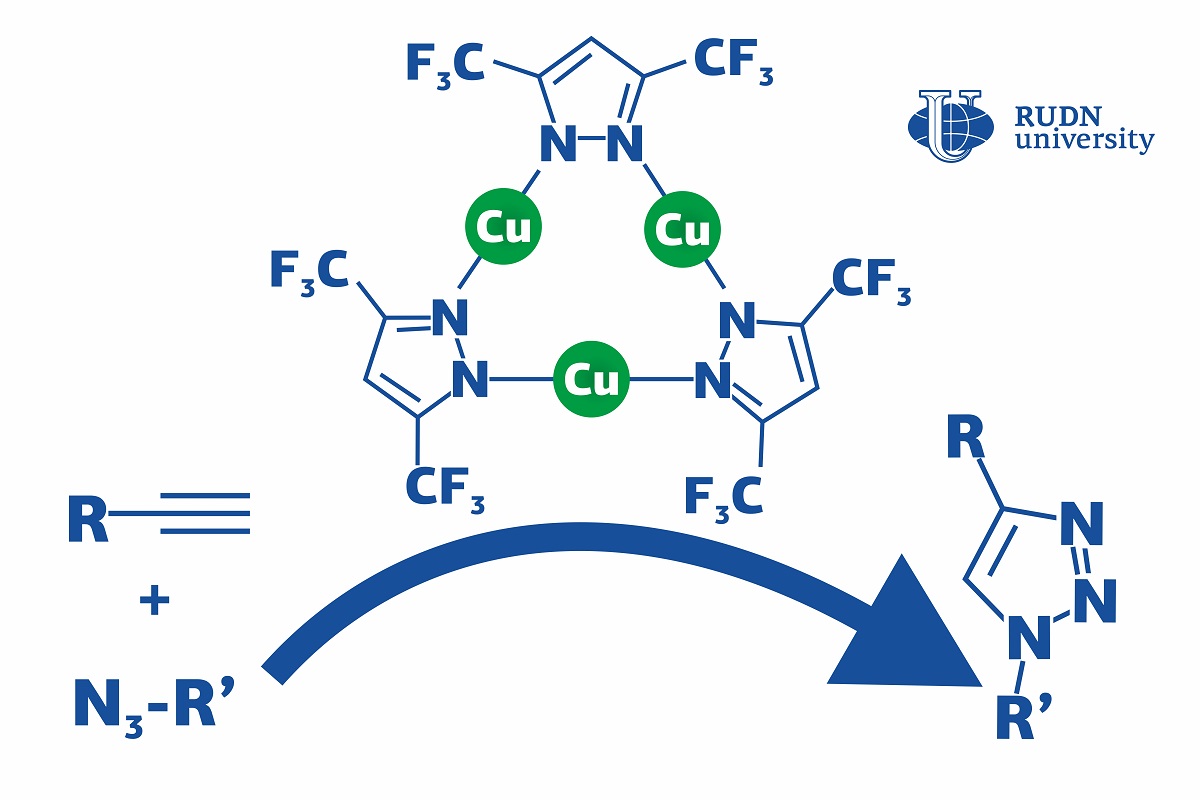
Click reactions are reactions in which simple molecules (or modules) 'click' together and assemble into a large complex molecule, just like details of a construction kit. They are mainly used in the pharmaceutical industry and polymer chemistry. Using the click reaction of the azide-alkine addition (CuACC), triazoles are obtained-biologically active substances with antibacterial, neuroleptic, antispasmodic activity — for example, fluconazole and itraconazole. Usually, copper-based catalysts are used for this. They speed up the process, but this requires additional conditions — for example, heating or additional reagents-chemicals. This increases the production cost. A chemist from RUDN University suggested using a copper complex, that accelerates the click reaction and lets it go on at room temperatures and without an additional base. Moreover, his team developed the first complete description of the reaction mechanism that had not been fully understood before, especially for different catalyst systems.
“CuAAC can involve different copper catalysts, but in many cases, they require severe conditions (such as high temperatures, additional reagents, and so on). Although the mechanism of this reaction has been thoroughly studied, the details of the catalysis are still being discussed," said Vladimir Larionov, a Candidate of Chemical Sciences, and a researcher at the Department of Inorganic Chemistry, RUDN University.
The team studied a chemical compound that had three copper ions bound with ligands (complex organic ions). The complex was used as a catalyst in the CuACC reaction at room temperature. Dichloromethane, toluene, and other substances were tested as solvents. However, even in their absence the copper complex let the team obtain the required reaction product in 4 hours, while without it the reaction did not happen at all. In the end, 99% of the initial substrate turned into triazole, and there were no by-products.
The team was also the first ever to study the mechanism of this reaction using mass spectrometry and quantum-chemical calculations. The molecules were broken down to charged fragments, and after that their structure was identified based on the mass to charge ratio of each fragment. The catalyst turned out to work in two ways at once: the ligand helped the alkyne lose a proton and enter an active state, while copper ions played a role in the formation of an intermediate. In the course of these processes, bonds were formed between metal ions in the catalyst and the particles of the substrates. Previously, the bond formation between the catalyst and the alkyne (i.e. copper acetilyde) had been considered the slowest step of the reaction. However, the team disproved this belief and confirmed that the formation of the first bond between two reagents sets up the rate of whole reaction depending on catalytic system.
"Our results show that the rate-determining step that determines the rate of the CuACC reaction depends on the catalyst system and reagents. Previously, this fact was underestimated enough," added Vladimir Larionov.
Article in the Journal of Catalysis.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.
The project to develop a cellular model of the placenta became the winner in the Scientific Materials category of the Young Scientists 3.0 competition, organized with the support of the Presidential Grants Foundation and T-Bank.
Ten scientific journals published by RUDN University have been included in the highest level of the state list of scientific publications, the White List.
Forests are not only the lungs of the planet, but also home to millions of species. However, it has remained unclear how underground interactions between trees and fungi affect forest species richness in different climatic conditions. Previous studies have yielded conflicting results: in some regions, the dominance of certain fungi reduced tree diversity, while in others it increased it.