New catalysts for the thermal-catalytic treatment of plastic waste developed at RUDN University
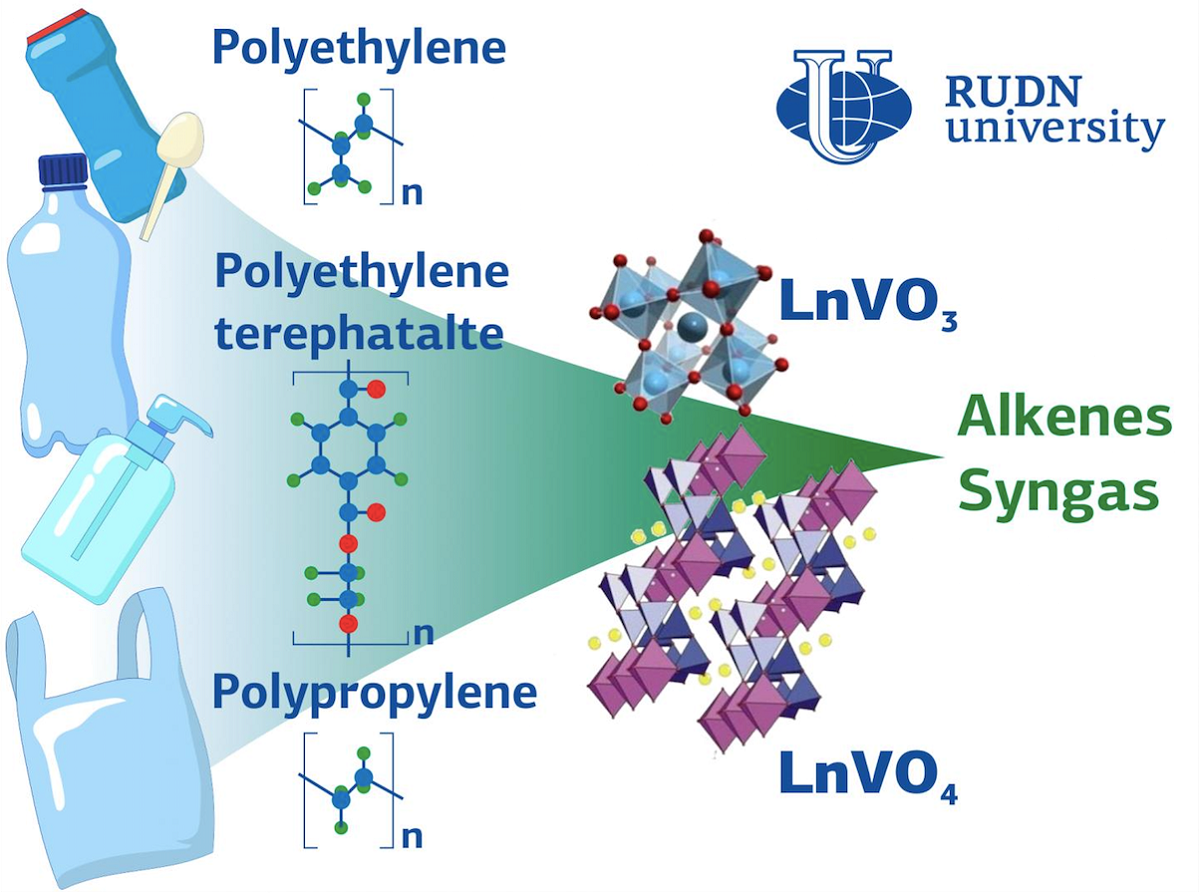
The treatment of plastic waste is an important issue for modern science and technology. Burning and burying the waste is ineffective: for example, while burning generates useful heat, it destroys the organic elements that could potentially be used in thermal-catalytic technologies. In the presence of certain catalysts, under moderate temperatures and pressure, and without oxygen, the decomposition of plastic could lead to the formation of numerous gases and liquids that in turn could be repurposed as raw materials for the synthesis of chemical products or motor fuels. RUDN University chemists conducted a series of experiments and confirmed that when used as catalysts, vanadates and vanadites of several rare-earth elements considerably increase the yield of light olefins. Moreover, they can radically change the composition of the end products of treatment.
“Most plastic materials do not biodegrade. Therefore, we require new technologies to treat plastic waste and reduce its negative environmental impact. One of the fields in which plastic waste could potentially be used is thermal-catalytic technologies. We actively work on the synthesis of new catalysts based on different REE compounds. We also study how they can be used to effectively turn polymer waste into promising chemical products. Our results show that this strategy could be practically implemented,” Alexander Cherednichenko, PhD, the Head of the Department of Physical and Colloid Chemistry at RUDN University.
In their experiment, the team studied four widespread types of plastic waste: polyethylene (PE), polypropylene (PP), polyethylene terephthalate (PET), and polyvinyl chloride (PVC). These materials are often used to produce industrial goods and products for everyday use. In the course of the experiment, ground plastic materials were loaded into a quartz reactor together with a catalyst and gradually heated in a nitrogen stream from 25? to 750?. The reaction products were analyzed and compared to the results of pyrolysis without a catalyst.
According to the team, vanadates and vanadites of rare-earth elements changed the composition of the gas products of pyrolysis. For example, around 5% of propylene was produced in the course of polyethylene pyrolysis without a catalyst, while in a thermal-catalytic process the yield increased to 15%. The pyrolysis of PET without a catalyst yielded about 75% of acetic aldehyde, while in the presence of certain catalysts the composition of the end products changed dramatically and the main reaction product was syngas (a mix of hydrogen and carbon monoxide), which, in turn, could potentially be used to generate chemical products or hydrogen for the energy sector. Therefore, the synthesized catalysts were able to regulate the composition of pyrolysis products of different types of plastic waste.
“Our study showed us a way to manage thermal-catalytic plastic waste treatment processes and obtain promising chemical products using vanadates and vanadites of rare-earth elements,” Ekaterina Markova, PhD, Dean of the Department of Physical and Colloid Chemistry at RUDN University.
The results of the study were published in the Catalysis Today journal.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.