The Chemist RUDN Proposed Effective Catalysts For Water Purification From Caffeine
Many industrial and household processes pollute water. Checking the water for chlorine or metal salts is a routine process, but some substances have started to get into the water recently, and it is still unknown how dangerous they are. For example, these are microplastic particles from cosmetics, drug residues and caffeine from beverages. Caffeine itself is not a poison. But it has a powerful effect on living organisms and can greatly distort the behavior and disrupt the health of aquatic inhabitants. In humans, accidental contamination of drinking water with caffeine can cause unpleasant consequences for the psyche. After all, this substance is a strong stimulant, it is not by chance that along with the food industry it is also used in pharmacology.
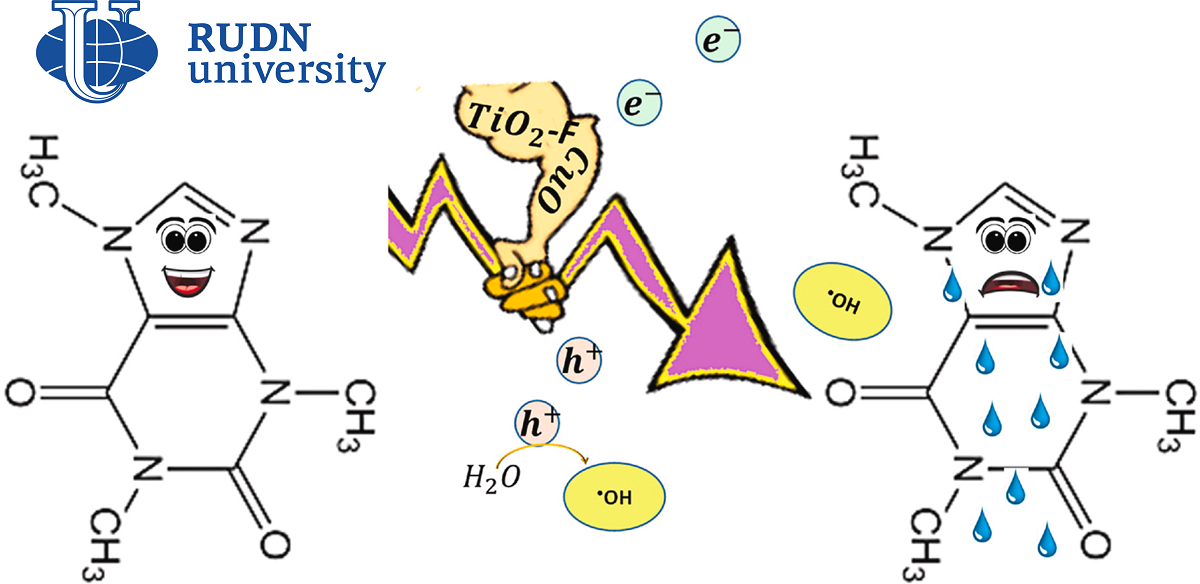
“To remove substances such as caffeine from water, there are different techniques: treatment with ozone or chlorine, filtration through membranes, absorption of pollutants by carbon. One of the simplest and most environmentally friendly methods is the use of photocatalysts, such as titanium dioxide. But in its pure form, this material does not have sufficient photocatalytic activity for industrial use,” Raphael Luque, PhD, head of the scientific center “Molecular Design and Synthesis of Innovative Compounds for Medicine”, RUDN.
The chemist RUDN conducted a number of experiments and improved titanium dioxide by fluoridation and the addition of copper and nickel oxides. First, the researcher prepared titanium dioxide powder with fluorine, and then fired at a temperature of up to 400 ° C to produce solid composites with metals from it. Although the content of copper and nickel oxides in them turned out to be small — less than 1% of the volume — the properties of the materials have changed. They have become less porous, and the size of the cells in their crystal lattice has increased.
The photocatalytic activity of the composites was compared with the efficiency of pure titanium dioxide. Aqueous solutions of caffeine and 100 milligrams of each catalyst were placed in one vessel and irradiated with ultraviolet light from a high-pressure mercury lamp. In addition, the caffeine solution was exposed to radiation without the participation of other chemicals. It turned out that up to 38% of caffeine in water decomposes under the action of ultraviolet light itself. Pure titanium dioxide increases this proportion to 54%, fluorinated — to 78%. Nickel and copper oxides showed even greater efficiency: in their presence, up to 88 and 90% of caffeine was destroyed, respectively.
“The addition of metals made the catalyst surface inhomogeneous. Under a transmission electron microscope, we can distinguish different facets in the crystal structure of composites, characteristic, for example, of copper oxide and titanium dioxide with fluorine. Different phases are in close contact and form heterojunctions: areas of a semiconductor where intense electron transfer occurs. This leads to the active formation of hydroxyl radicals -OH, which destroy caffeine,” — Rafael Luque, PhD, head of the scientific center “Molecular Design and Synthesis of innovative compounds for Medicine” RUDN.
The differences in the structure of the material significantly increased the photocatalytic activity of the composites. The reaction rate in the presence of catalysts with nickel and copper was 4.2 times higher than when using only titanium dioxide, and 2.8 times higher compared to fluorinated dioxide. The experiment showed that composites can be used several times in a row without a significant decrease in activity. Therefore, the method proposed by the RUDN chemist is not only environmentally friendly, but also potentially economically effective.
The results are published in the journal Chemosphere.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.