Scientific seminar of post-doc Kletskov A.V. « Novel small macrocycles on the basis of bis-furfurylsulfamides»
The constant growth of interest in different macrocycles can be mainly explained by their broad scope of possible applications. Macrocycles were proven to be valuable bioactive substances and complexing agents. Among those, furan-containing macrocycles stand out as both valuable intermediates for the synthesis of other macrocyclic substances through convenient transformations, such as Diels-Alder reaction, as well as promising bioactive compounds. Herein we report a novel approach to the synthesis of small furan macrocycles, containing pharmacophore arylsulfamides group based on the reaction of N-benzyl-1,5,3-dioxazepane – an effective electrophile for double Mannich reactions (scheme 1).
Scheme 1
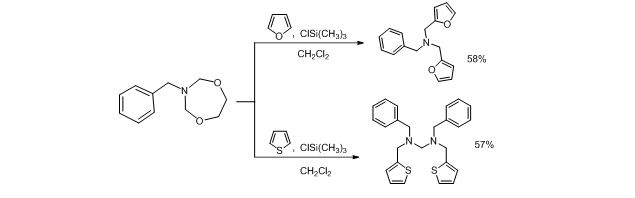
Corresponding macrocycles are both promising as bioactive substances as well as intermediates in organic synthesis. On the initial stage of our research we found out that the reaction of N-benzyl-1,5,3-dioxazepane proceeds differently in the case of unsubstituted furan and thiophene (scheme 2).
Scheme 2
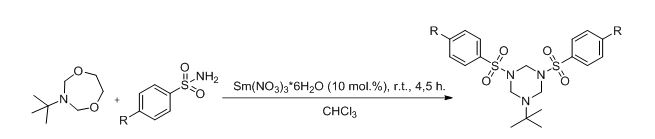
As by-product of our main research a novel approach to synthesis of N,N’,N’’- substituted hexahydrotriazine was developed (scheme 3).
.JPG)