RUDN University Chemist Creates Reusable Vanadium Catalysts on a Carbon Framework
Complexes with vanadium are used in the chemical industry as catalysts, for example, to produce sulfuric or adipic acid. Vanadium catalysts give a good yield of the final product when heated, but after the reaction they cannot be separated from other reagents, so it is impossible to use the catalyst again. The chemist of RUDN University together with colleagues from Portugal received two vanadium catalysts and fixed them on carbon nanoscale frameworks. This increased the efficiency of the catalysts and made it possible to reuse them almost without any quality loss. In addition, the new catalysts allow the reaction to be carried out in more green conditions — using microwave radiation instead of oil bath heating method.
“The use of carbon materials as supports has additional advantages, given their tunable texture and surface chemistry, able to fit the envisaged applications” said Armando Pombeiro, professor at RUDN University.
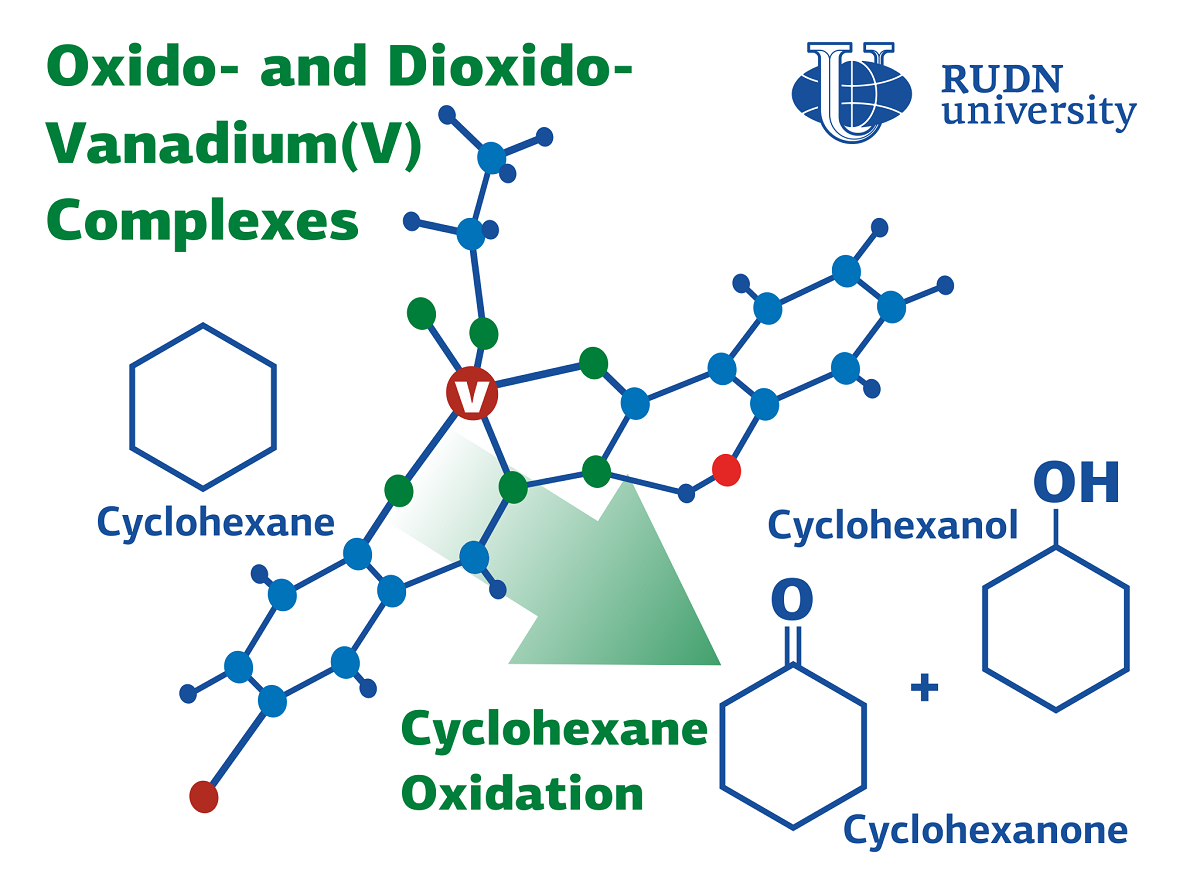
Chemists have obtained two complexes with vanadium oxide and dioxide from aroylhydrazone. Its structure was studied using elemental analysis, Fourier spectroscopy, nuclear magnetic resonance and spectrometry. The catalytic properties of the compounds were evaluated in the oxidation reaction of cyclohexane. Then chemists put the vanadium complexes on carbon frameworks made of activated carbon and carbon nanotubes and studied the catalytic activity again. The reaction was carried out under mild conditions in an acetonitrile solution, a 70% solution of tert-butylhydroperoxide was used as an oxidizer. Microwave radiation was used to heat the reagents — for the first time for this type of reaction. At the output of the reaction, a mixture of cyclohexanol and cyclohexanone, intermediates for the production of adipic acid, was obtained.
The efficiency of catalysts fixed on carbon was 1.5–2 times higher than that of “free” ones. In this case, catalysts with a carbon framework can be used several times. To separate the catalyst from other substances, it is enough to filter, rinse and dry the reaction mixture. After that, the catalyst is ready for use again. After four reaction cycles, the efficiency decreased by only 30%.
“The immobilization of complexes resulted in an increase in the catalyst efficiency and allowed catalyst recyclability for up to four consecutive cycles, although with some loss of activityЭто было невозможно для однородных ванадиевых комплексов. We trust this work will open a plethora of future applications for new immobilized vanadium (and other metal) complexes, for the oxidation of different alkanes (apart from cyclohexane) and other catalytic reactions, using MW irradiation”, said Professor Armando Pombeiro.
The results are published in Nanomaterials.
A RUDN agrotechnologist has identified wheat genotypes that are resistant to a dangerous fungal pathogen that infects plants even before the snow melts and reduces yields.
RUDN University engineers have calculated the parameters of a system that can prevent lunar power plants from overheating. These developments will be needed when planning for long-term lunar missions and colonizing the satellite.
Landfills are the third largest source of anthropogenic methane in the world. They account for ~11% of estimated global emissions. Methane is 80 times more powerful than carbon dioxide and is the second largest driver of man-made climate change. Scientists from around the world met at Zhejiang University's Hangzhou campus to determine the best available technologies for recovering energy and materials from non-recyclable residual waste.