RUDN University chemist proposed new approach to the synthesis of the ABCD ring system of alpkinidine
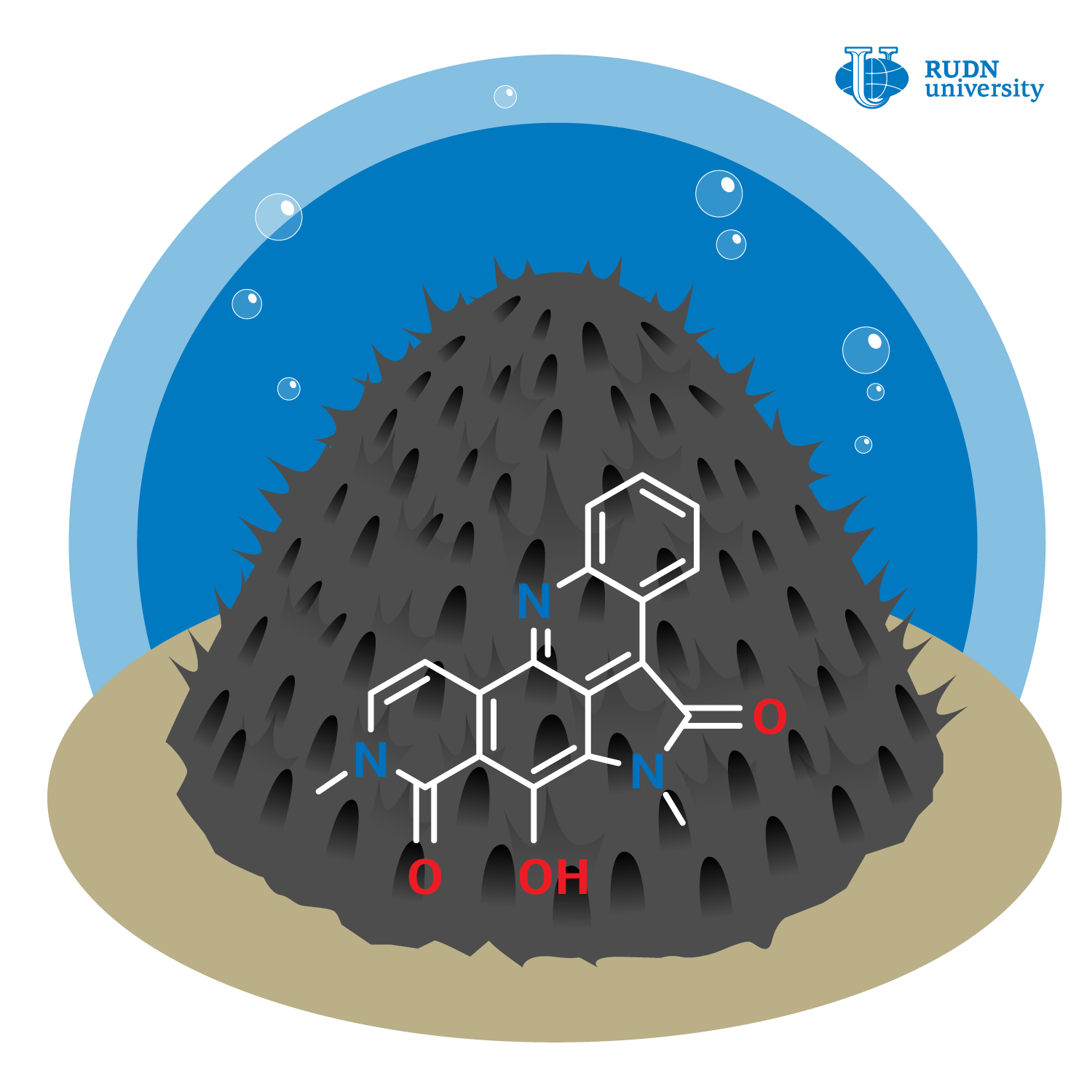
Alpkinidine is a naturally occurring purple substance obtained from the sea sponge Xestospongia cf. carbonaria. A specific feature of its structure is the presence of a complex ABCDE five-rings system. Alpkinidine is not yet well studied, in contrast to the closely related amphimedine and neoamphimedine. These substances are known to demonstrate antiviral, antiparasitic, as well as antifungal and antibacterial activity. It is also known about neoamphimedine that it can show selective cytotoxicity to the cell lines of solid tumors, i.e., tumors developed not from the cells of the haematogenic system. Neoamphimedine undergoes preclinical trials and could be an effective anticancer drug in the future.
The antitumor effect of neoamphimedine is due to its inhibitory effect on DNA topoisomerase – an enzyme that is responsible for regulating the topology of DNA. The development of anti-cancer drugs — topoisomerase inhibitors — is a promising field in pharmacology, since compounds with similar inhibitory properties show a very strong antitumor effect. Most of them are not used in therapy because of the strong toxic effect or unpredictable side effects in the physiological environment, so scientists are actively searching for analogs with similar useful properties and without a negative effect on the human body.
Alpkinidine is similar in structure to neoamphimedine, so it is assumed that it may demonstrate a similar range of biological properties, including powerful antitumor activity. However, the method of complete synthesis of the substance was not previously developed. The synthesis of similar analogs of alpkinidine in structure would help in the development of an effective scheme for the synthesis of alpkinidine itself.
Fedor Zubkov, Ph.D. in Chemistry, Associate Professor of the Department of Organic Chemistry of RUDN University, synthesized an analog of alpkinidine and thus developed a new approach to the synthesis of compounds with an ABCD ring system of alpkinidine.
First, chemists have replaced the E-ring of alpkinidine with the phenyl ring. At the same time, the system of five connected rings was not disrupted, so that the properties characteristic of alpkinidine were preserved. The synthesis of the analog of alpkinidine was carried out in several stages. The chemists obtained an intermediate compound that was synthesized with a yield of 94% using the Negishi reaction, a method for synthesizing complex organic compounds using palladium catalysts. Then it was treated with sodium ethoxide and then using alkylation obtained a target analog of alpkinidine with a yield of 88%.
As a result of this work, a method for the complete synthesis of compounds with the ABCD ring system of alpkinidine was developed on the basis of the synthesis of an analog of alpkinidine. In the future, scientists plan to use the developed method for the synthesis of alpkinidine. In the absence of negative effects on the human body, drugs based on it can become an integral part of effective anti-tumor therapy.
The article is published in ChemistrySelect
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.