Iron Is to Blame for Carbon Dioxide Emissions from the Soil, Says a Soil Scientists from RUDN University
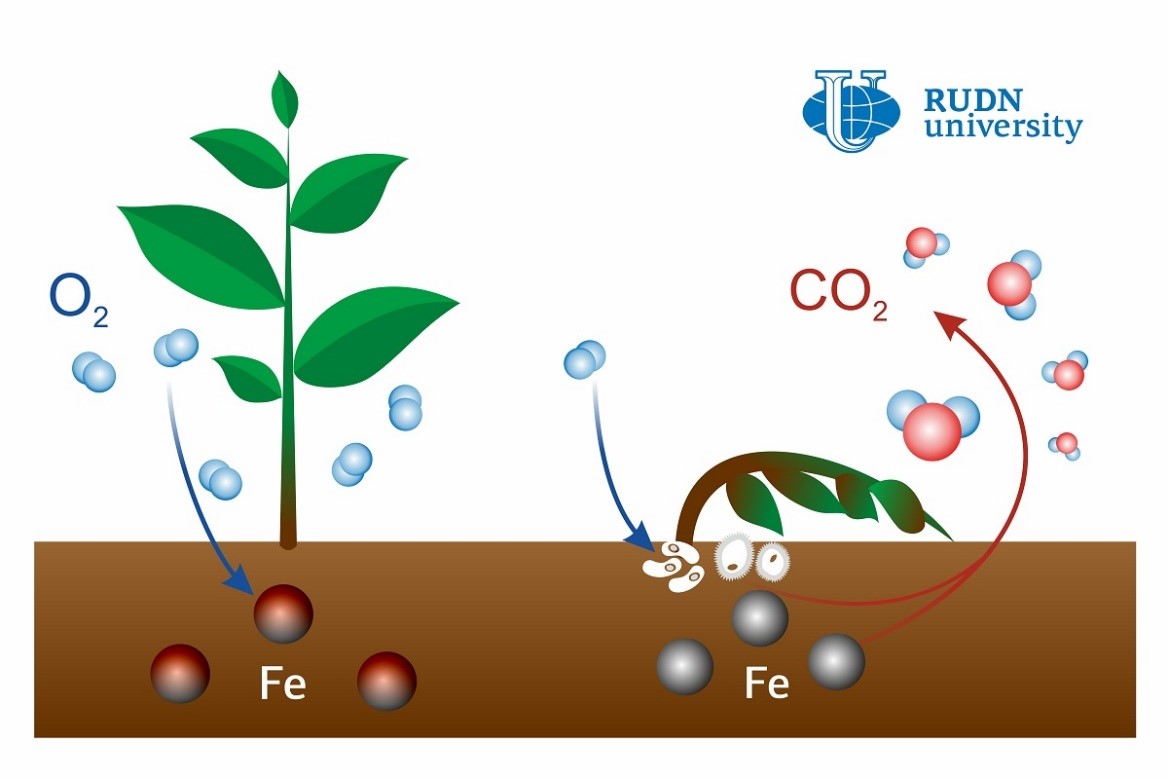
Carbon dioxide is considered one of the main reasons for global warming, and almost half of it is released to the atmosphere from the soil. The most active ‘soil breathing’ areas usually contain decomposing plant waste. Such areas tend to have hotspots: local zones up to 1 cm3 in volume where the decomposition process is almost 100 times faster. Due to a combination of high moisture and good aeration, these hotspots offer perfect living conditions to soil microorganisms. Previously, microbial activity had been considered the reason for active CO2 emissions. However, a soil scientist from RUDN University confirmed that it was in fact oxidation-reduction transitions of soil iron that caused them.
“Previously we used to believe that the reason for carbon dioxide emissions from hotspots were microorganisms that treated plant waste with special enzymes and turned it into gas. However, we demonstrated that a big part of this process was due not only to enzymes. Iron facilitates the formation of active oxygen forms (radicals) that affect insoluble organic matter, destroy it, and turn it into the soluble state,” said Yakov Kuzyakov, a PhD in Biology, and the Head of the Center for Mathematical Modeling and Design of Sustainable Ecosystems at RUDN University.
The scientist also pointed out that microorganisms accelerate plant waste decomposition only several times, not hundreds of times. In the course of their activity bacteria release hydrogen peroxide that can react with iron and take one electron from it, creating active oxygen forms, i.e. substances with one free unpaired electron. This type of oxygen is chemically active and quickly oxidizes organic matter, causing its destruction.
To confirm that active oxygen forms cause CO2 emissions, the team created an artificial hotspot. To do so, they put 300 g of soil in a container, added plant waste (straw), and a solution of iron sulfate. The moisture level in the container was kept at 90%. The team measured the volume of CO2 emitted from the container for 40 days and then compared the results to measurement data collected from soils without iron and straw, and with low (45%) moisture levels. The sample in the container turned out to release 22 mg of CO2 per 1 kg of soil, which was approximately 22 times more than the volume released by low-moisture soil and soil without plant waste. After that, the team measured the changes in the volume of iron, carbon, hydrogen peroxide, and active oxygen forms in the vicinity of the hotspot. Based on the ratio of all these substances the team concluded that iron reacted with hydrogen peroxide and produced active oxygen.
“Unlike earlier theories, ours is focused on the primary role of active oxygen forms. It is a simple scenario. At first, plant waste stimulates the growth of bacteria. When there are enough bacteria, they consume almost all oxygen. These are perfect conditions for the concentration of iron which otherwise would oxidize. Then the iron reacts with hydrogen peroxide, and organic matter is decomposed under the influence of active oxygen forms. In its decomposed state it attracts even more bacteria, and the process intensifies several times,” added Yakov Kuzyakov.
The study was the first one to confirm that biological processes in soil hotspots are driven by the activity of free radicals (i.e. substances with a free electron). According to the team, this mechanism might also take place in other hotspots, for example, those found in the rhizosphere (the soil around plant roots). In the future, this data can be used to reduce CO2 emissions from the soil.
The results of the study were published in the Geoderma journal.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.