RUDN Chemists Received an Antibiotic from Chitin
Chemical modification of chitosan allows to obtain water-soluble chitosan derivatives with increased antibacterial activity. In this study, chitosan derivatives with extremely high antibacterial activity and pronounced water solubility were obtained. To synthesize chitosan derivatives that form highly active nanoparticles, scientists have developed a new approach based on a combination of click chemistry and ultrasonic processing methods. Using the proposed approach, it will be possible to obtain other polysaccharide particles with antibacterial activity. The work is published in the International Journal of Biological Macromolecules.
Chitosan is a non-toxic, biodegradable and biocompatible polymer that is industrially obtained from chitin by removing the acetyl group from its links. Chitosan is actively used as a dietary supplement, cosmetic agent and growth regulator in agriculture, added to animal feed. However, all the useful properties of chitosan are related to its adhesive properties: it interacts with the mucous membranes, facilitating the penetration of drugs into the body. Chitosan is characterized by weak antibacterial activity, which is strongly limited by its low solubility in water.
RUDN University chemists under the guidance of Assistant Professor of the Department of Inorganic Chemistry Andrey Kritchenkov for the first time obtained derivatives with antibacterial properties at the level of modern antibiotics. Increased antibacterial activity was found to be characteristic of chitosan compounds with a triazole cycle and a betaine fragment, in which the number of cationic groups can be controlled.
To obtain this compound, Andrey Kritchenkov and his colleagues first used an original technique-it combines two approaches that were relatively recently used for chemical transformations of chitosans. The first of them is azide-alkine cycloaddition, one of the most important methods of click chemistry, which allows very selectively and with high yield to bond the desired molecules together. The second approach is ultrasonic treatment, which significantly accelerates the click reaction and does not require anaerobic conditions for its implementation. Using these two methods simultaneously, scientists could produce a cationic polymer while controlling its size and precise chemical composition.
‘We first brought to the area of chemistry of chitosan simultaneous combination of click reactions and ultrasonic treatment and was able to pick up such conditions of ultrasonic irradiation when the reaction proceeds faster and conditions of its implementation much smoother and easier, and a polymer chain of the original chitosan retains its integrity, that is not broken. Apparently, the complexity of optimizing the conditions for frequency, power, amplitude of ultrasound stopped the attempts of our predecessors to finish this job. This is a very complex and painstaking job’, Andrey Kritchenkov said.
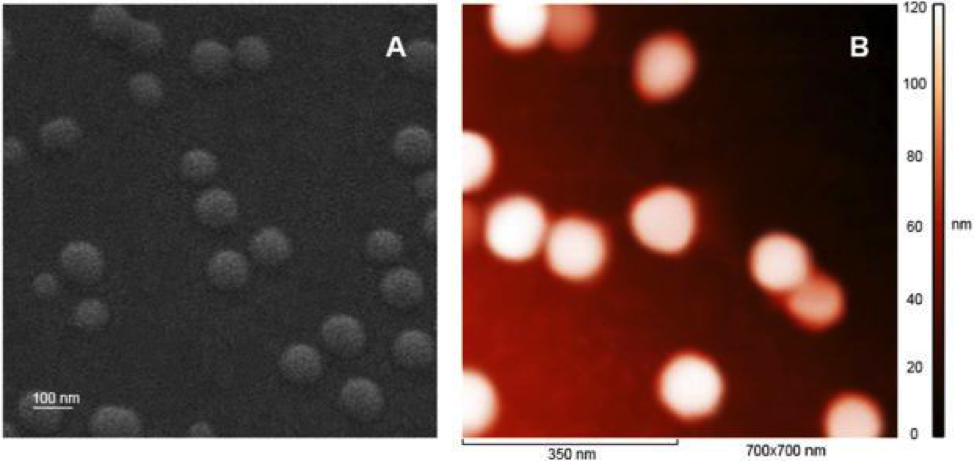
Then, to increase the antibacterial activity of the polymer, chemists obtained nanoparticles with a diameter of about 100 nanometers from individual polymer molecules. It is known that polymers often acquire antibacterial properties in the form of nanoparticles. Andrey Kritchenkov and his colleagues checked the presence of required properties in nanoparticles on the cells of Staphylococcus aureus (Staphylococcus aureus) and E. coli (Escherichia coli). It turned out that for individual components of the polymer compound – triazole, betaine, and chitosan – the inhibition zone did not exceed 13 mm, for the obtained nanoparticles this value reached 45 mm for Staphylococcus and 36 mm for E.coli. This, for example, is more than one and a half times higher than the standard numbers for antibiotics – ampicillin and gentamicin.
The authors note that the application was found not only for nanoparticles of chitosan derivatives but also for the polymer in its original form. Polymer molecules are polycations, so they effectively bind polyanions, such as nucleic acids.
Therefore, they can be used for transfection – the injection of DNA into eukaryotic cells by a nonviral method. Scientists measured transfection activity on human liver cells and obtained values of about 30 thousand cells per square centimeter – that is, at the level of modern commercial drugs such as lipofectin.
‘The perspective of application as an antibacterial agent is definitely there. Now our colleagues-biologists are finishing experiments in vivo and they are very successful’, scientist noted.
According to scientists, the main advantage of the obtained chitosan derivatives as an antibacterial agent and for genetic information transfer systems is the absence of toxic effects. Chemists believe that in the same way, it will be possible to obtain other polymer particles with antibacterial and transfection activity.
The article in the International Journal of Biological Macromolecules
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.