A chemist from RUDN University came up with a new catalyst for fuel synthesis
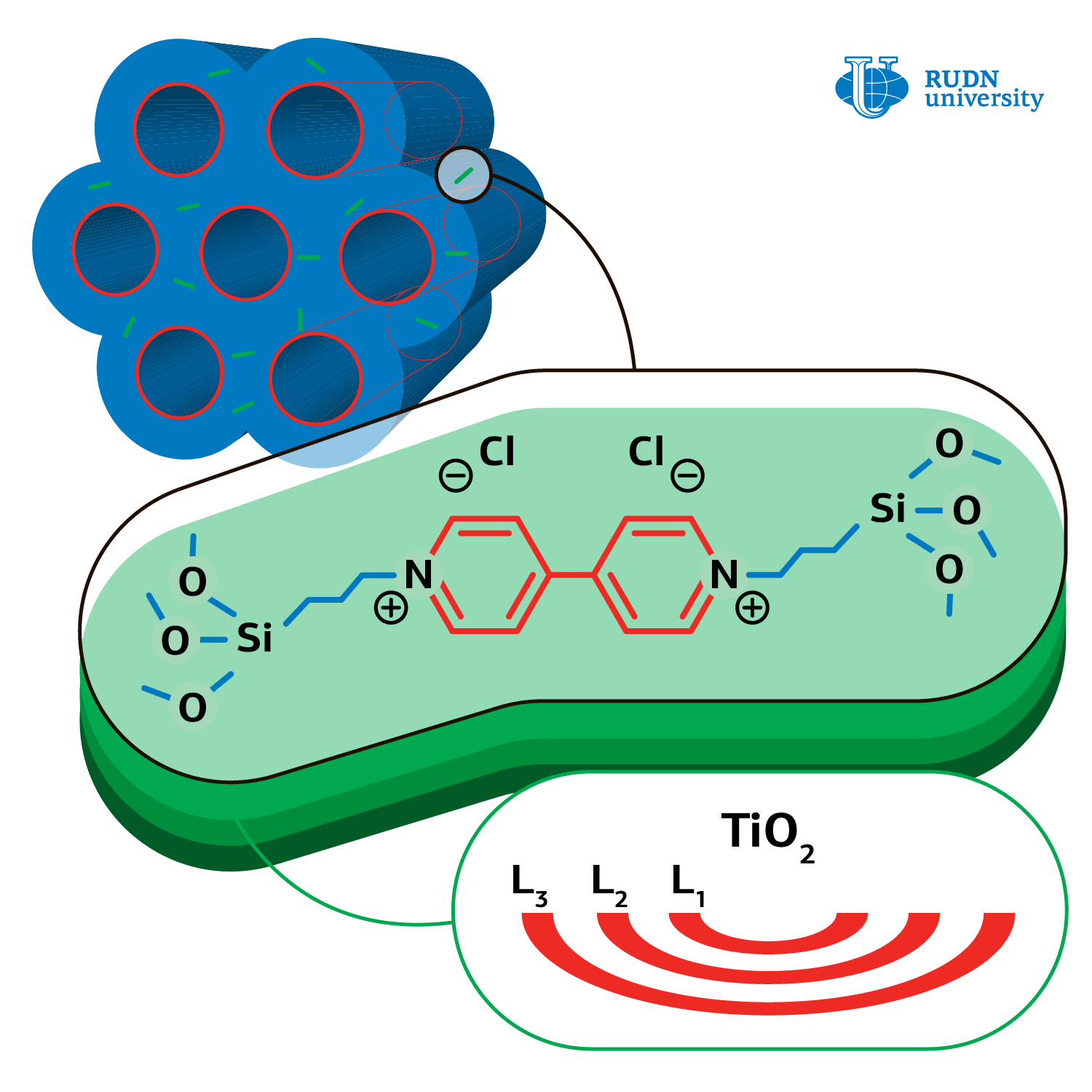
Hydrogen energetics development is not possible without methods of hydrogen secure storage and generation. Formic acid is a non-toxic and highly stable source of hydrogen. Н2 and СО2 are obtained by decomposition of acid under light irradiation on catalysts’NPs, the role of which is fulfilled by platinum, palladium, and other costly metals compounds. Chemists decided to test what the products of formic acid photo‑oxidation will be obtained if the cheaper layered amorphous oxide of titanium is used as a catalyst.
Professor Rafael Luque, United Institute of Chemical Research RUDN and his colleagues from Iran, Spain, China, and South Korea synthesized a catalyst — titanium amorphous oxide based on organic-silicate matrix. At the beginning the chemists obtained mesoporous (2-50 nm) matrix material, where bridging dimeric groups of organic viologen compound presented. Later the precursor — titanium butoxide was loaded, followed by matrix drying at 60 о С and its transformation into amorphous titanium oxide.
The chemists performed the reaction of formic acid oxidation in different conditions: different temperature regimes (from 25 to 60о С) and different quantity of catalyst from amorphous titanium oxide (from 5 to 20 mg), with different solvents (water, ethanol, methanol, and others). The results of experiments showed that the quickest reaction proceeds under UV radiation, in water and at room temperature, providing only CО2 and Н2О. Neither hydrogen, nor carbon monoxide, which poison any photo‑oxidation catalyst, were identified in products. Such products formation is due to the catalyst’s non-crystalline structure. In their work the authors came up with the photo-oxidation mechanism and specified basic stages.
The authors also found out that viologens improve the quality of catalyst, because it generates electron-proton vapors in photocatalysis, thus extending the lifetime of the catalyst. The catalyst can be easily reprocessed and reused at least in four cycles without noticeable ageing.
The scientists made an input in fundamental chemistry development, having investigated a new mechanism of formic acid formation. The results of the present research will allow to minimize risks and expenditures in common use of this type of catalyst in future development.
The article is published in the journal ChemCatChem.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.