RUDN University chemist synthesized a coordination polymer iron with a derivative of nicotinic acid
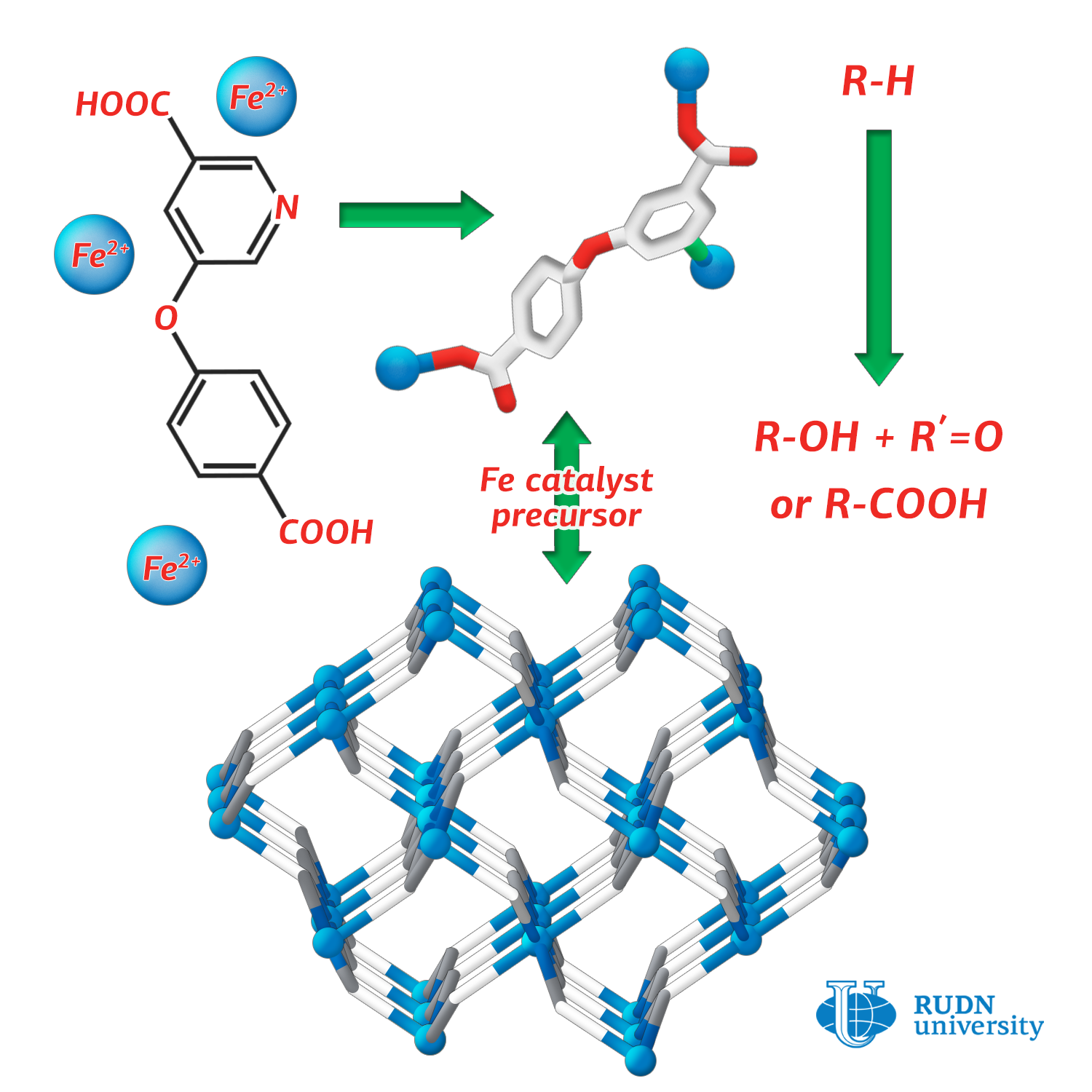
Coordination polymers are compounds that consist of a metal atom and surrounding organic ligands. They are often more stable than pure organic substances. RUDN chemist Alexander Kirillov used a nicotinic acid derivative (H2cpna) as a building block in the synthesis, and iron atoms played the role of the metal center. Substituted nicotinic acid can act as a ligand — it contains one phenyl and one pyridine ring, which are linked by an ether functional group.
To synthesize the polymer, the reaction was performed under hydrothermal conditions between iron (II) sulfate in water and H2cpna at 160 ⁰C. The synthesis lasted three days. X-ray diffraction analysis and other methods were used to confirm the structure and characteristics of the obtained substance.
The catalytic activity of the substance for different reactions was studied. Alexander Kirillov from RUDN conducted the processes of oxidation and carboxylation of propane and cyclic alkanes (saturated hydrocarbons closed in a cycle) under mild conditions. The reaction yield was 23%. For comparison, in the industrial process of oxidation of cyclohexane to cyclohexanol and cyclohexanone (products that are used in the production of plastics), the yield is only 5-10%.
The study of the catalytic activity of the polymer obtained by RUDN chemists showed that it can be used to catalyze the processes of oxidative functionalization of saturated hydrocarbons, and provide a greater yield for the reaction under mild conditions.
Article in Crystals magazine.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.