RUDN University chemist synthesized a new compound with strong anti-diabetic properties
In the small intestine, the complex structure of starch is broken down into simpler oligosaccharides by the action of the enzyme α-amylase. Then, under the action of the enzyme α-glucosidase, the resulting oligosaccharides pass into glucose and other monosaccharides. If you inhibit — that is, “turn off” — one or both of these enzymes, the rate of carbohydrate absorption will decrease, and, consequently, the concentration of glucose in the blood will decrease. This antidiabetic effect makes researchers pay more and more attention to the search and synthesis of α-amylase and α-glucosidase inhibitors.
Yuness El Bakri from the RUDN University in collaboration with colleagues 17 have synthesized new derivatives of 1,2,4-triazole. They belong to the class of heterocycles — organic compounds that contain “rings” of carbon atoms and atoms of other elements. Due to their structure, which resembles that of natural products, heterocyclic compounds have interesting biologically active properties.
RUDN chemists studied the structure of new heterocyclic compounds using x-ray diffraction analysis and spectral methods. They then evaluated in vitro the inhibitory activity of all 1,2,4-triazole derivatives and compared them with acarbose, a hypoglycemic drug that inhibits α-glucosidase. All of the new compounds proved to be potent inhibitors of α-glucosidase, and three of them also demonstrated the ability to inhibit α-amylase.
Using molecular docking, a method for modeling the properties of molecules, the researchers showed that three triazole derivatives — 6 — methyl-7H,8H,9H-[1,2,4]triazolo[4,3-b][1,2,4]triazepine-8-one and 6-methyl-7H-[1,2,4]triazolo[4,3-b][1,2,4]triazepine-8(9H)-thion-surpass acarbose in inhibitory activity relative to α — glucosidase.
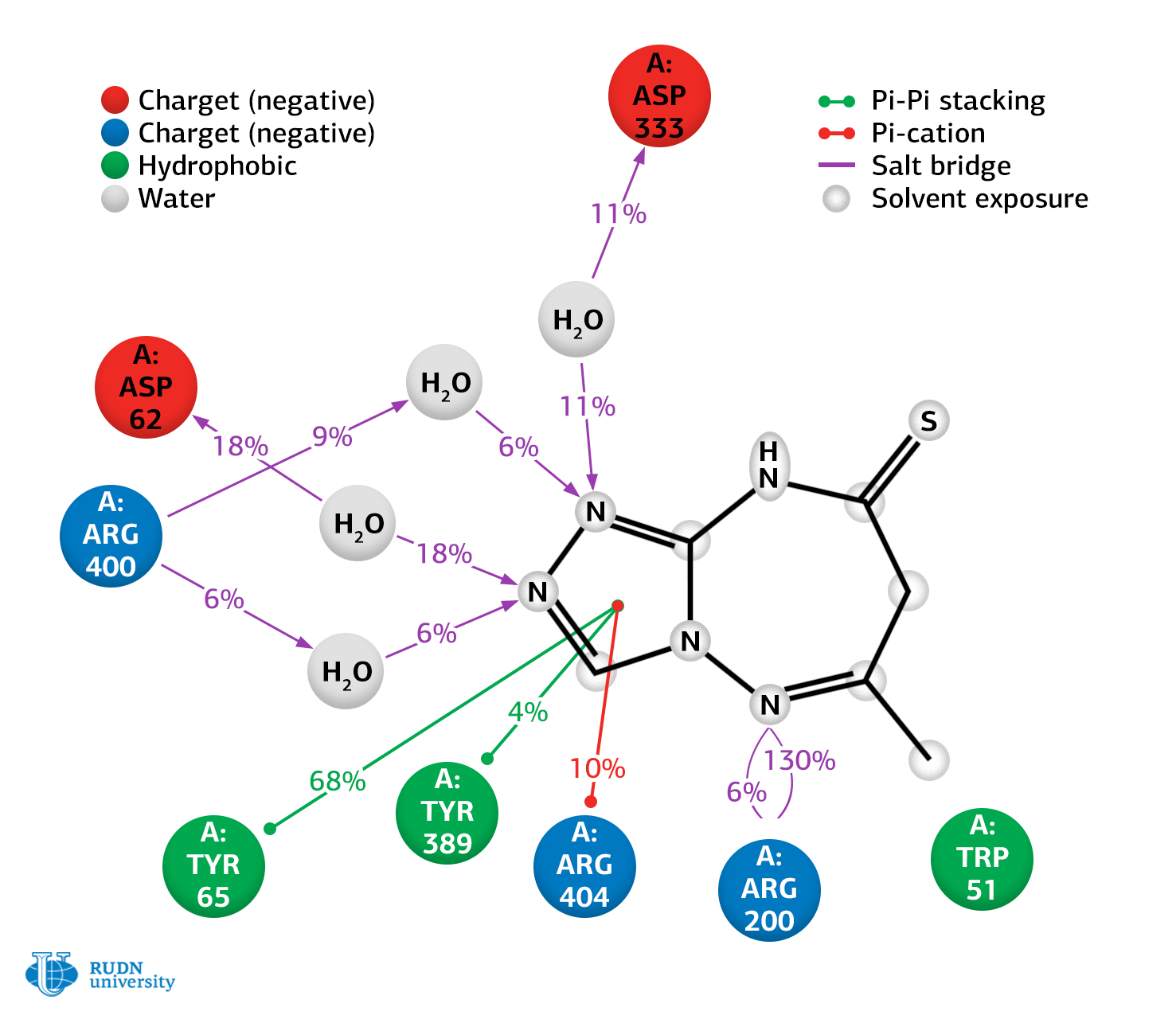
Based on the results of experiments, RUDN biochemists selected the best conformation of 6-methyl-7H-[1,2,4]triazolo[4,3-b][1,2,4]triazepine-8(9H)-thion in complex with α-glucosidase and performed its molecular dynamic modeling.
Calculations have shown that the degree of inhibition of α-glucosidase is mainly due to the number and strength of bonds between the compound and the active residues of the enzyme. The results obtained allow us to conclude that the synthesized compound is stable.
Younes El Bakri and his colleagues also showed the antioxidant properties of the new compounds. Chemists used a spectrophotometer to measure changes in the optical density of solutions containing specifically colored free radicals (ABTS cation-radical (2,2’-azinobis3-ethylbenzothiazoline-6-sulfonate) and DPPH radical (2,2-diphenyl-1-picrylhydrazyl)), to which antioxidants were added. Using these methods, they determined the ability of the antioxidant to interact with ABTS and DPPH radicals. Also, the antioxidant activity of 1,2,4-triazole derivatives was evaluated by evaluating the ability of antioxidant iron reduction.
The chemists concluded that the 1,2,4-triazole derivatives they obtained are promising for use as antidiabetic drugs. They expect that the properties of these substances will soon become the subject of Toxicological and pharmacological studies in vivo.
Article in the journal Bioorganic Chemistry.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.
Matilda Pavlovna Mityaeva was born in 1925. In November 1942, she volunteered for frontline duty. She participated in the Great Patriotic War from November 1942 to June 1945 as part of the 53rd Infantry Division of the 475th Infantry Regiment. She was wounded twice.
The team led by Sergey Zyryanov, Head of the Department of General and Clinical Pharmacology, became the winner of the All-Russian competition of scientific projects "Technologies for Human Health".
RUDN University constantly adapts to the changes of the modern world and responds to challenges flexibly. This allows us to keep the standard of a world-class research university. The sphere of science is no exception. Peter Dokukin, Head of the Research Division, presented the updated R&D Programme at the meeting of the RUDN University Academic Council.