A molecular "merry-go-round" complex will make OLED screens twice as bright
Displays with organic LEDs differ from other modern types of displays, plasma and LCD, in higher brightness, contrast and lower power consumption. However, OLED displays are more expensive. Also, the raw material for their production – conductive polymers – is toxic, and it creates difficulties in production and disposal.
To reduce the cost of OLED displays and get rid of toxic raw materials, it is possible to use fluorescent complex compounds — molecules where small organic fragments surround the central ion of the metal instead of polymers. But so far, it has not been possible to find complexes that would show a clear advantage in brightness and efficiency over polymers. Sufficiently effective compounds based on iridium or platinum were very expensive, and cheaper complexes with transition metal ions were not effective enough.
RUDN University chemist Alexander Smolyakov, an employee of the Scientific Center "Cluster of target synthesis of natural compounds", found compounds that would make OLED displays much brighter and more economical than polymer ones. The centers of these complexes were not platinum or iridium, but cheaper copper and silver, which also proved to be more effective and less toxic compared to polymers.
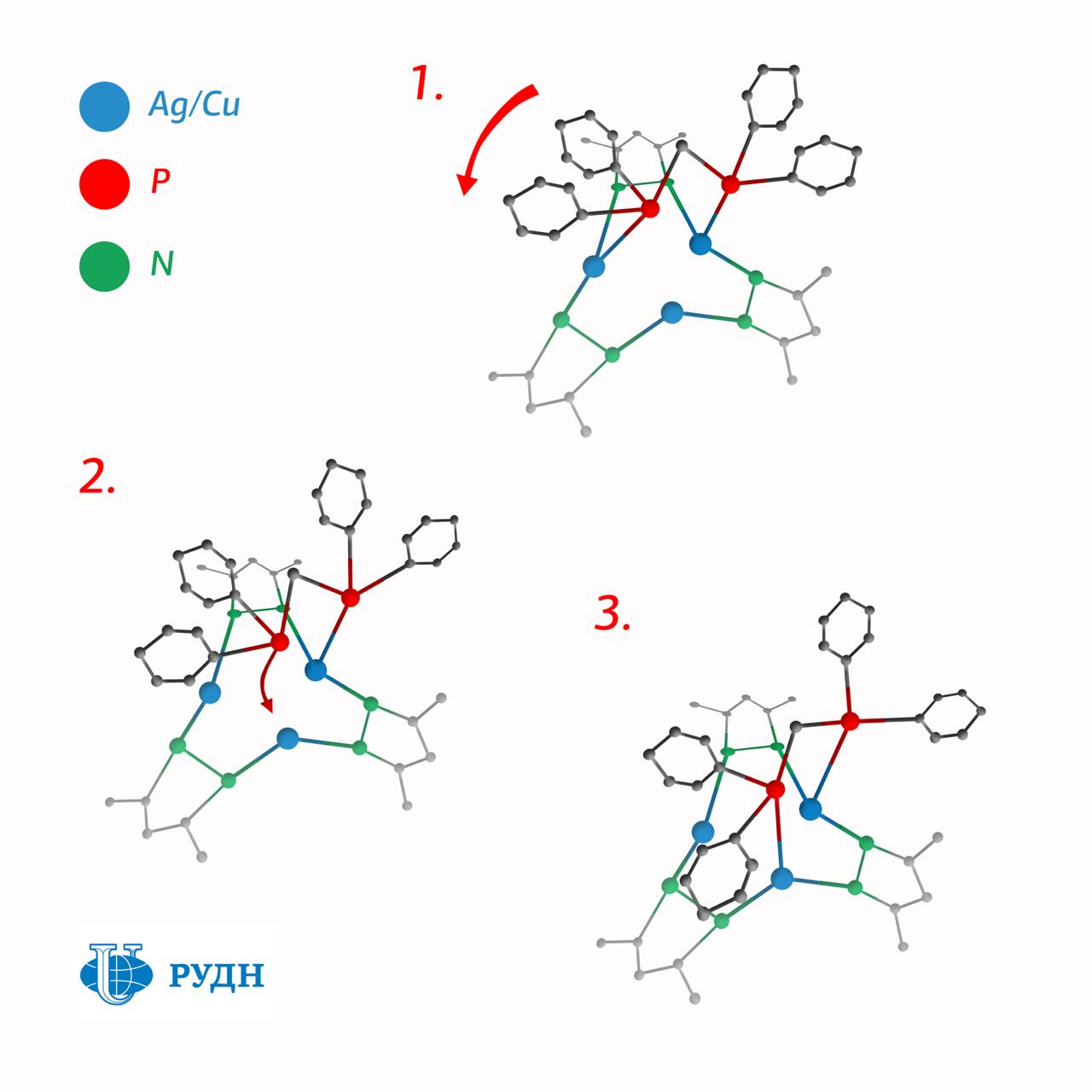
The researcher synthesized a molecule with not one but three ions of monovalent copper or silver in the center. To make this structure of three metal ions stronger, the chemist stabilized it using derivatives of pyrazole — aromatic molecules with two nitrogen atoms in the cycle, and used organophosphorus molecules as ligands (molecules — electron donors surrounding an ion). In this case, the ions of monovalent copper and silver form a three-center nucleus in the form of a triangle, and ligands join the nucleus through phosphorus atoms and remain quite mobile.
At room temperature, the energy of thermal oscillations is sufficient to break the bond between phosphorus and metal for a short time. However, there are two phosphorus atoms in a molecule, and there are three metal atoms. So one of the metal atoms always is without a pair and if there is a single phosphorus atom, the metal atom attracts it immediately. That is, the ligand "jumps" to the neighboring ion in the three-center nucleus and forms a bond that can soon be destroyed again due to thermal fluctuations.
The molecule thus turns into a kind of molecular "merry-go-round". This configuration makes stable complexes with nuclei of silver ions, and complexes with nuclei of monovalent copper — compounds do not decay immediately after synthesis, like many other structures of this type.
Chemists have found that such "merry-go-round" structure of complex compounds leads to the emergence of two energy states, the transition between which can lead to luminescence. In the case of copper, this structure has a significant quantum yield – that is, the ratio of the number of absorbed and emitted photons is 41 percent. The organic polymers that are used in OLED displays today provide a quantum yield twice as low: about 20 percent.
Scientists think that three-center copper "merry-go-round" complexes are promising for the future of OLED technology.
The article in Inorganic Chemistry
Products derived from microalgae represent a cutting-edge development in the field of bioeconomy. The potential of this biological resource was discussed at the international research seminar “Foundations for a Green Sustainable Energy”, part of the BRICS Network University’s thematic group on “Energy”. The event was organized by the Institute of Ecology at RUDN University.
Ambassadors of Russian education and science met at a conference in RUDN University to discuss how they can increase the visibility of Russian universities and research organizations in the world, and attract more international students in Russia.
The international scientific seminar hosted by RUDN Institute of Ecology “Experience of participation in student organizations as a way to form career skills” united scholarship recipients of the International Student Mobility Awards 2024 and Open Doors, along with members of the scientific student society “GreenLab” and the professional student association “Kostyor (Bonfire)” shared their projects focused on environmental protection.