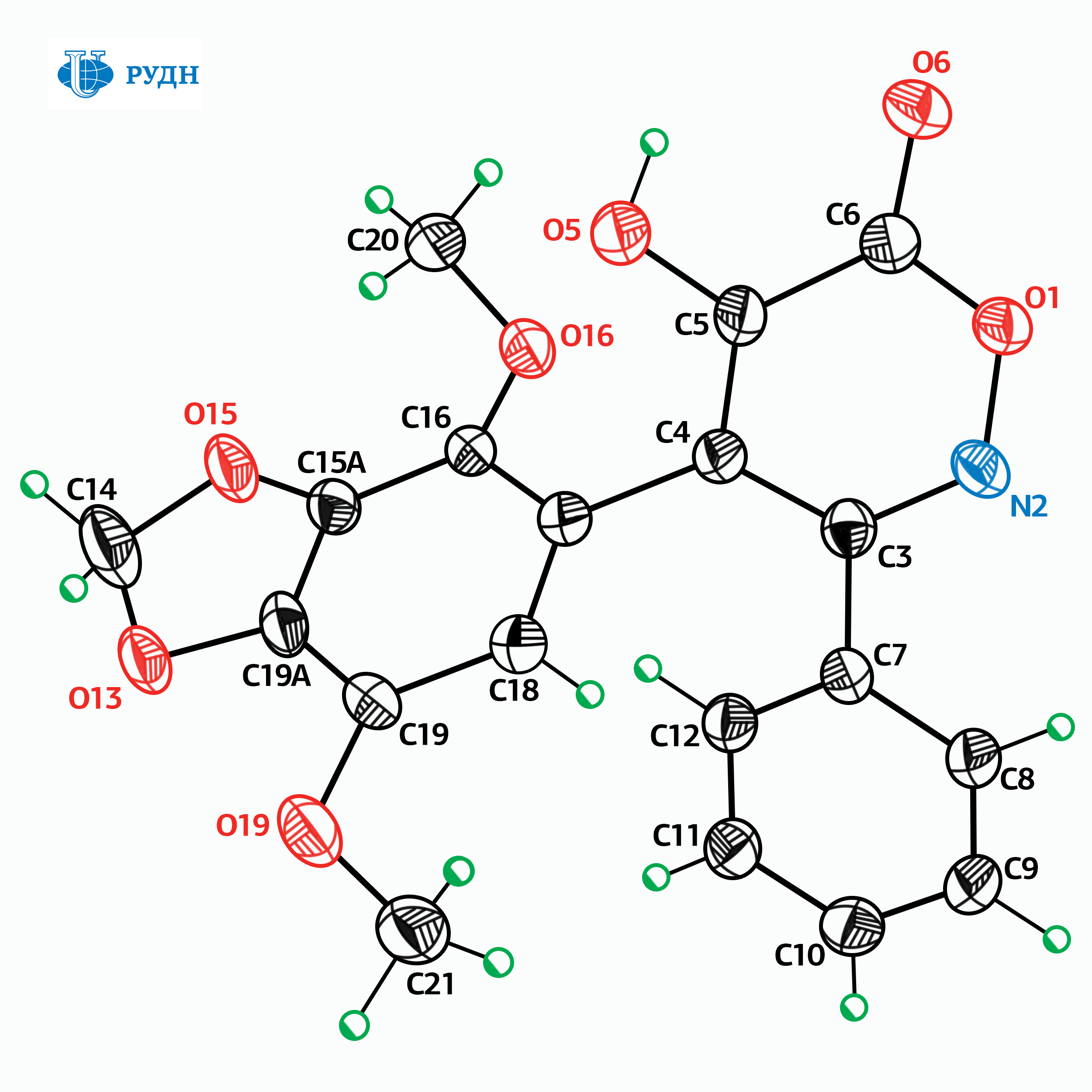
RUDN chemist, along with colleagues from the RAS Institutes, simplified the synthesis of antitumor compounds
Many modern anticancer drugs are toxic, difficult to access and/or very expensive. Moreover, tumor cells may develop resistance to the drugs used. Therefore, researchers study the biological properties of molecules in order to obtain new antitumor drugs with optimal properties. One of the common approaches to the search for such drugs is testing of analogues of substances that already showed antitumor activity. Such substances include, in particular, isoxazole derivatives that inhibit — “turn off” — the Hsp90 protein, which is necessary for the survival of tumor cells. However, compounds of this class are inaccessible due to the complexity of the synthesis procedure, which requires, in particular, the complete absence of water molecules, and the reagents are expensive and toxic.
RUDN chemist Viktor Khrustalev and his colleagues developed a method for the synthesis of isomers of these substances, that is, the compounds that are identical in atomic composition but different in the arrangement of atoms in space. Easily accessible derivatives of arylnitromethanes and chloroacetamides were used as raw materials, and the reaction itself was carried out at temperatures not exceeding 80 degrees at atmospheric pressure and did not require anhydrous conditions.
The obtained substances had anticancer activity, but unlike the prototype compounds, they did not inhibit Hsp90 protein. Their mechanism of action is based on the destabilization of the cell division process as they prevent the formation of microtubules, which are important in the process of cell division.
Taxol derivatives, one of the most commonly used antitumor agents, have the same mechanism of action. Basing on the compounds obtained by the scientists, it is possible to create a substitute for an expensive, inaccessible and highly toxic derivatives of taxol in the treatment of cancer.
The work was published in European Journal of Organic Chemistry.
Products derived from microalgae represent a cutting-edge development in the field of bioeconomy. The potential of this biological resource was discussed at the international research seminar “Foundations for a Green Sustainable Energy”, part of the BRICS Network University’s thematic group on “Energy”. The event was organized by the Institute of Ecology at RUDN University.
Ambassadors of Russian education and science met at a conference in RUDN University to discuss how they can increase the visibility of Russian universities and research organizations in the world, and attract more international students in Russia.
The international scientific seminar hosted by RUDN Institute of Ecology “Experience of participation in student organizations as a way to form career skills” united scholarship recipients of the International Student Mobility Awards 2024 and Open Doors, along with members of the scientific student society “GreenLab” and the professional student association “Kostyor (Bonfire)” shared their projects focused on environmental protection.