RUDN University chemist discovered a way to quadruple the speed of toluene photooxidation
Toluene is a combustible liquid that is extracted from gasoline fractions of oil for the production of paint materials, chemicals, solvents, and aviation fuel. Benzaldehyde and carbon dioxide are produced during the photooxidation of toluene. Benzaldehyde is used to produce benzoic acid, an important element in the production of food preservatives, medicines, and chemical raw materials. However, the widespread industrial photooxidation of toluene with oxygen into benzoic acid is a slow process. RUDN University chemist proposed a new way to control the photooxidation of toluene using catalysts g-C3N4/TiO2 and manganese (Mn), increasing the reaction rate 4.3 times and increasing the efficiency of benzaldehyde production.
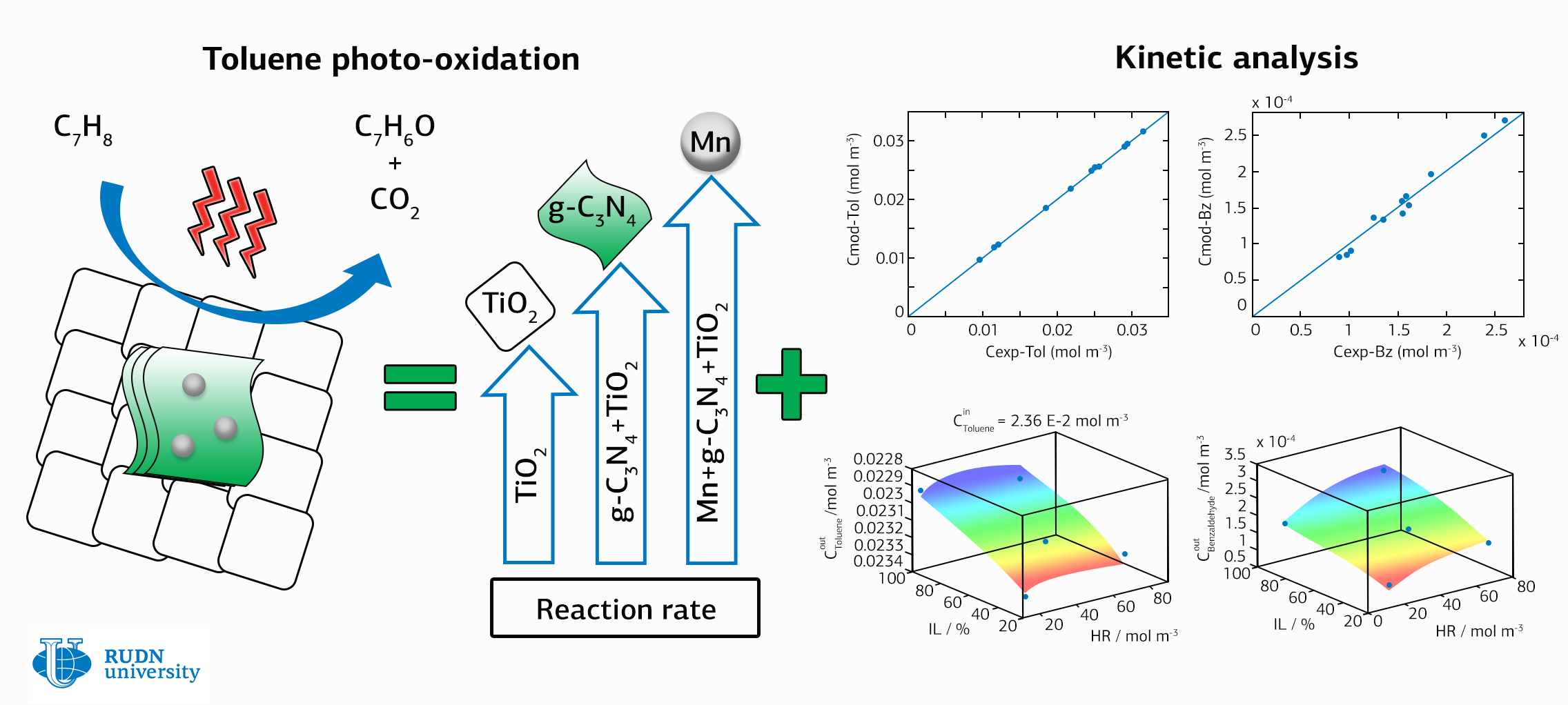
Rafael Luque from the Research Institute of Chemistry of RUDN University notes that only benzaldehyde and carbon dioxide are found as reaction products for all solids without significant differences between composite samples. And their reference catalyst based on titanium dioxide is highly active and converts the partial oxidation product mainly into benzaldehyde, increasing the efficiency of the reaction.
The chemists conducted a combination of adsorption and kinetic studies. They developed evidence that the reaction occurs by a hydroxyl-mediated mechanism – it means that the increase in the activity of the elements depends on changes in the rate of formation of hydroxyl particles that are present on the surface of solids.
Scientists have found that the reaction rate is quantitatively related to the rate of production of hydroxyl particles, which interact with toluene affect the process of photooxidation. This means that regulating the contact between the components allows controlling the rate of photoactivity.
Scientists found that the new catalysts increase the reaction rate of toluene photooxidation by about 2.5 times, and the addition of manganese to the carbon nitride component shows a further increase in the reaction activity by 1.8 times. That is, the contact between the components of the composite catalyst g-C3N4/TiO2 significantly improves the photooxidation of toluene, and manganese enhances such a beneficial effect.
Thus, chemists have found that the contact between the components allows you to control the rate of photoactivity by slowing or accelerating the process of photooxidation of toluene. The authors of the study report that the findings refute the previously existing hypothesis in the scientific literature about the nature of the photooxidation process.
Rafael Luque, Director of the Scientific Center of the Research Institute of Chemistry of RUDN University, conducted research in collaboration with colleagues from leading institutions in Spain (universities of Madrid, Granada, Cordoba).
The article is published in the Chemical Engineering Journal.
Products derived from microalgae represent a cutting-edge development in the field of bioeconomy. The potential of this biological resource was discussed at the international research seminar “Foundations for a Green Sustainable Energy”, part of the BRICS Network University’s thematic group on “Energy”. The event was organized by the Institute of Ecology at RUDN University.
Ambassadors of Russian education and science met at a conference in RUDN University to discuss how they can increase the visibility of Russian universities and research organizations in the world, and attract more international students in Russia.
The international scientific seminar hosted by RUDN Institute of Ecology “Experience of participation in student organizations as a way to form career skills” united scholarship recipients of the International Student Mobility Awards 2024 and Open Doors, along with members of the scientific student society “GreenLab” and the professional student association “Kostyor (Bonfire)” shared their projects focused on environmental protection.