The new protocol will allow to obtain bioactive compounds bypassing by-products
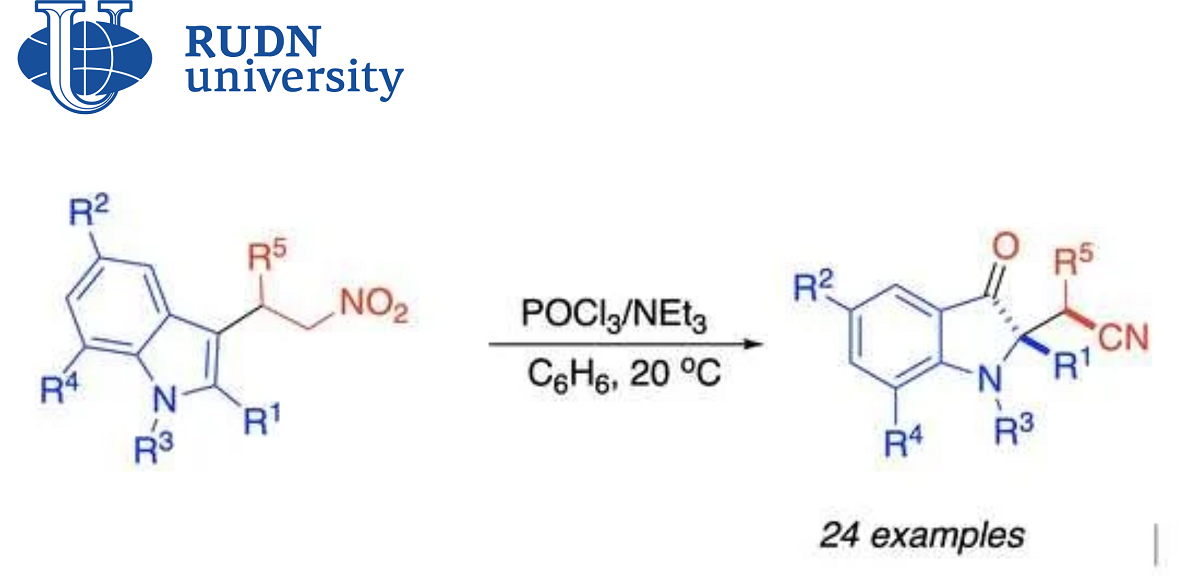
Derivatives of indolylacetic acid (for example, indole-acetonitriles) are found in nature and have a wide range of biological activity. Their synthetic analogues are created in the laboratory for the production of medicines. Recently, a new method for the synthesis of indole-acetonitriles has been discovered — spirocyclization of nitroalkens. However, this approach has a drawback — an inert by-product is obtained in the process, which reduces the overall efficiency of synthesis. A group of chemists from the North Caucasus Federal University, RUDN and the University of Kansas (USA), led by Doctor of Chemical Sciences Alexander Aksenov, found a solution to this problem.
“Derivatives of 1H-indole-2-acetic acid are key auxiliary compounds of natural and synthetic pharmaceuticals. Therefore, the methods of obtaining them remain in the focus of attention of many research groups. The recently discovered [4+1]-spirocyclization of nitroalkens into indoles has given a new convenient approach to the production of indole-acetonitriles. However, it is complicated by the formation of inert by-products. We offer a solution to this problem, which makes it possible to efficiently convert unwanted by-products into the required acetonitrile molecules,” Candidate of Chemical Sciences Elena Sorokina, Associate Research Professor of the Department of Organic Chemistry of the RUDN.
Chemists have improved the protocol that was proposed earlier. Now the final product is obtained directly from the predecessor, without a side intermediate compound. To do this, the scientists had to sort through several auxiliary substances and find the necessary reaction conditions.
Chemists found out that an acidic environment was a necessary condition for the appearance of an unnecessary by-product. And for the synthesis of the final indole-acetonitrile, on the contrary, a weakly basic medium is needed. Therefore, chemists were looking for the ratio and composition of acid and base, as well as solvent and temperature, which would allow byproduct to be bypassed. The optimal set turned out to be phosphorus oxychloride, triethylamine, solvent benzene and a temperature of 20℃. Such conditions provided a 72% yield of the final indole-acetonitrile, bypassing the by-product. Chemists also tried to change the starting substances and obtained other similar indole-acetonitriles with a yield of up to 81% according to the new protocol. The spectrum of biological activity of the obtained compounds has yet to be clarified, but chemists suggest that they may be useful for creating new drugs.
“We used the new methodology to synthesize a small targeted library of nitriles. Now our laboratories are already conducting a study of the biological activity of these new compounds,” Candidate of Chemical Sciences Elena Sorokina, Associate Research Professor of the Department of Organic Chemistry of the RUDN.
The results are published in the journal Molecules
A RUDN agrotechnologist has identified wheat genotypes that are resistant to a dangerous fungal pathogen that infects plants even before the snow melts and reduces yields.
RUDN University engineers have calculated the parameters of a system that can prevent lunar power plants from overheating. These developments will be needed when planning for long-term lunar missions and colonizing the satellite.
Landfills are the third largest source of anthropogenic methane in the world. They account for ~11% of estimated global emissions. Methane is 80 times more powerful than carbon dioxide and is the second largest driver of man-made climate change. Scientists from around the world met at Zhejiang University's Hangzhou campus to determine the best available technologies for recovering energy and materials from non-recyclable residual waste.